Page 294 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 294
THE HALOGENS
274
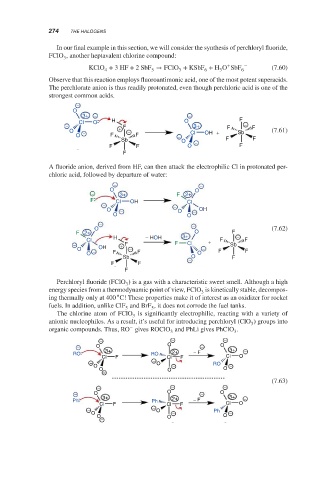
In our final example in this section, we will consider the synthesis of perchloryl fluoride,
FClO , another heptavalent chlorine compound:
3
+
KClO + 3HF + 2 SbF → FClO + KSbF + H O SbF 6 − (7.60)
6
3
4
5
3
Observe that this reaction employs fluoroantimonic acid, one of the most potent superacids.
The perchlorate anion is thus readily protonated, even though perchloric acid is one of the
strongest common acids.
−
O
3+ − −
Cl O H O F
− F 3+ F − F
O + − Cl OH + Sb (7.61)
O − F F −
Sb O F F
F F O − F
F
A fluoride anion, derived from HF, can then attack the electrophilic Cl in protonated per-
chloric acid, followed by departure of water:
− −
O O
− 3+ F 2+
F Cl OH Cl
− −
O − O OH
O O −
− −
O (7.62)
F 2+ O F
H − HOH 3+ −
Cl + F F
− + F F Cl Sb
O OH − − F F
O − F F O
Sb O F
F F −
F
Perchloryl fluoride (FClO ) is a gas with a characteristic sweet smell. Although a high
3
energy species from a thermodynamic point of view, FClO is kinetically stable, decompos-
3
∘
ing thermally only at 400 C! These properties make it of interest as an oxidizer for rocket
fuels. In addition, unlike ClF and BrF , it does not corrode the fuel tanks.
5 5
The chlorine atom of FClO is significantly electrophilic, reacting with a variety of
3
anionic nucleophiles. As a result, it’s useful for introducing perchloryl (ClO ) groups into
3
−
organic compounds. Thus, RO gives ROClO and PhLi gives PhClO .
3
3
− − −
− O O − O 3+
RO 3+ RO 2+ − F −
Cl F Cl F Cl O
− − O RO
O − −
O O O
−
(7.63)
− − −
− O O − O
3+ 2+ 3+ −
Ph Ph − F
Cl F Cl F Cl O
− − O Ph
O − −
O O O
−