Page 290 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 290
THE HALOGENS
270
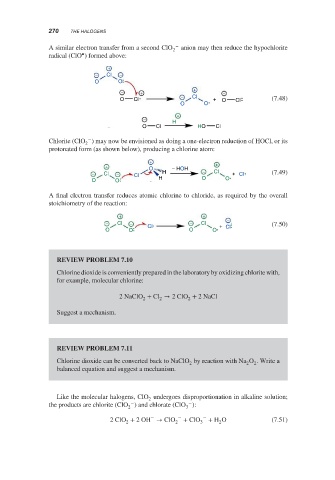
−
A similar electron transfer from a second ClO 2 anion may then reduce the hypochlorite
•
radical (ClO ) formed above:
+
− Cl −
O O
− + + −
O Cl − Cl + O Cl (7.48)
O O
+
−
H
O Cl HO Cl
−
Chlorite (ClO ) may now be envisioned as doing a one-electron reduction of HOCl, or its
2
protonated form (as shown below), producing a chlorine atom:
+
+ +
O − HOH
− Cl − Cl H − Cl + Cl (7.49)
O O H O O
A final electron transfer reduces atomic chlorine to chloride, as required by the overall
stoichiometry of the reaction:
+ +
− Cl − Cl − Cl + − (7.50)
O O O O Cl
REVIEW PROBLEM 7.10
Chlorine dioxide is conveniently prepared in the laboratory by oxidizing chlorite with,
for example, molecular chlorine:
2NaClO + Cl → 2ClO + 2NaCl
2
2
2
Suggest a mechanism.
REVIEW PROBLEM 7.11
Chlorine dioxide can be converted back to NaClO by reaction with Na O . Write a
2 2 2
balanced equation and suggest a mechanism.
Like the molecular halogens, ClO undergoes disproportionation in alkaline solution;
2
−
−
the products are chlorite (ClO ) and chlorate (ClO ):
2 3
− − −
2ClO + 2OH → ClO 2 + ClO 3 + H O (7.51)
2
2