Page 291 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 291
7.7 HEPTAVALENT CHLORINE 271
−
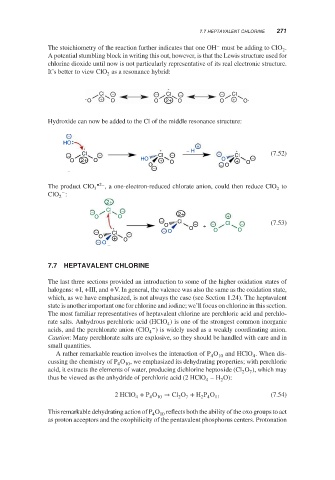
The stoichiometry of the reaction further indicates that one OH must be adding to ClO .
2
A potential stumbling block in writing this out, however, is that the Lewis structure used for
chlorine dioxide until now is not particularly representative of its real electronic structure.
It’s better to view ClO as a resonance hybrid:
2
Cl − − Cl − − Cl
O + O O 2+ O O + O
Hydroxide can now be added to the Cl of the middle resonance structure:
−
HO
+
− H
− Cl − Cl − − Cl (7.52)
O 2+ O HO + O O + O −
O − O
−
The product ClO 3 •2– , a one-electron-reduced chlorate anion, could then reduce ClO to
2
−
ClO :
2
2+
− Cl − 2+
O O +
− Cl
O O − + − Cl − (7.53)
− Cl − − O O O
O +
− O O
7.7 HEPTAVALENT CHLORINE
The last three sections provided an introduction to some of the higher oxidation states of
halogens: +I, +III, and +V. In general, the valence was also the same as the oxidation state,
which, as we have emphasized, is not always the case (see Section 1.24). The heptavalent
state is another important one for chlorine and iodine; we’ll focus on chlorine in this section.
The most familiar representatives of heptavalent chlorine are perchloric acid and perchlo-
rate salts. Anhydrous perchloric acid (HClO ) is one of the strongest common inorganic
4
−
acids, and the perchlorate anion (ClO ) is widely used as a weakly coordinating anion.
4
Caution: Many perchlorate salts are explosive, so they should be handled with care and in
small quantities.
A rather remarkable reaction involves the interaction of P O and HClO . When dis-
4 10 4
cussing the chemistry of P O , we emphasized its dehydrating properties; with perchloric
4 10
acid, it extracts the elements of water, producing dichlorine heptoxide (Cl O ), which may
2 7
thus be viewed as the anhydride of perchloric acid (2 HClO –H O):
4 2
2HClO + P O 10 → Cl O + H P O 11 (7.54)
2 4
4
4
7
2
This remarkable dehydrating action of P O reflects both the ability of the oxo groups to act
10
4
as proton acceptors and the oxophilicity of the pentavalent phosphorus centers. Protonation