Page 298 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 298
THE HALOGENS
278
As you’ll see, the mechanism is not particularly complex, but there are a few subtle points
that are worth appreciating. The role of the radical initiator provides a natural starting point
for our discussion. The very weak O–O bond (BDE ∼138 kJ/mol) cleaves homolytically
under the influence of heat or light:
O
O
Ph O
Ph O C +
C O Ph C C (7.74)
O Ph
O
O
The benzoyloxy radical formed may cleave further, producing phenyl radicals and highly
stable CO :
2
O
Ph O
C Ph + C (7.75)
O O
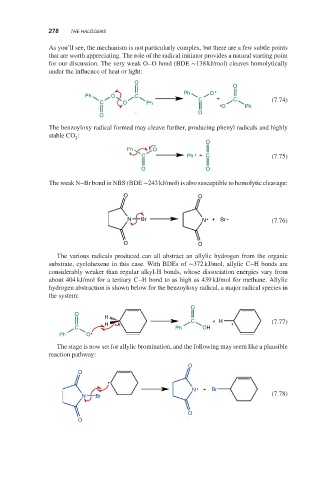
The weak N–Br bond in NBS (BDE ∼243 kJ/mol) is also susceptible to homolytic cleavage:
O O
N Br N + Br (7.76)
O O
The various radicals produced can all abstract an allylic hydrogen from the organic
substrate, cyclohexene in this case. With BDEs of ∼372 kJ/mol, allylic C–H bonds are
considerably weaker than regular alkyl-H bonds, whose dissociation energies vary from
about 404 kJ/mol for a tertiary C–H bond to as high as 439 kJ/mol for methane. Allylic
hydrogen abstraction is shown below for the benzoyloxy radical, a major radical species in
the system:
O
O
H
C + H (7.77)
H
C Ph OH
Ph O
The stage is now set for allylic bromination, and the following may seem like a plausible
reaction pathway:
O
O
N + Br
N Br (7.78)
O
O