Page 302 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 302
THE HALOGENS
282
O −
O X O X
C X
R C C C C H + C −
R R O
− HO X X X X
HO X X (7.86)
O X O X
C C − C + H C
H −
R O X R O
X X X
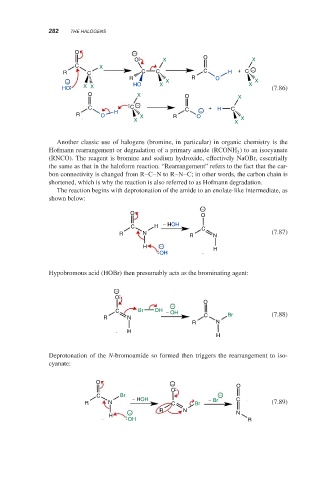
Another classic use of halogens (bromine, in particular) in organic chemistry is the
Hofmann rearrangement or degradation of a primary amide (RCONH ) to an isocyanate
2
(RNCO). The reagent is bromine and sodium hydroxide, effectively NaOBr, essentially
the same as that in the haloform reaction. “Rearrangement” refers to the fact that the car-
bon connectivity is changed from R–C–N to R–N–C; in other words, the carbon chain is
shortened, which is why the reaction is also referred to as Hofmann degradation.
The reaction begins with deprotonation of the amide to an enolate-like intermediate, as
shown below:
−
O
O
C H − HOH C
R N R N (7.87)
H −
H
OH
Hypobromous acid (HOBr) then presumably acts as the brominating agent:
−
O
O
−
C Br OH − OH
R N C Br (7.88)
R N
H
H
Deprotonation of the N-bromoamide so formed then triggers the rearrangement to iso-
cyanate:
O −
O
O
C Br −
R N − HOH C Br − Br C (7.89)
− R N N
H
OH R