Page 307 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 307
3
7.11 -IODANES 287
Somewhat regrettably, we will skip a proper mechanistic discussion of iodonium
ylide-mediated group transfer chemistry. As mentioned, the majority of such processes
require transition-metal catalysis, a topic outside the scope of this book.
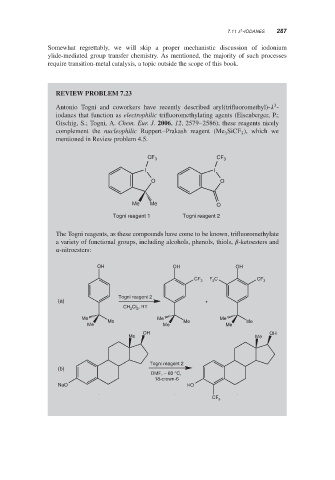
REVIEW PROBLEM 7.23
3
Antonio Togni and coworkers have recently described aryl(trifluoromethyl)- -
iodanes that function as electrophilic trifluoromethylating agents (Eisenberger, P.;
Gischig, S.; Togni, A. Chem. Eur. J. 2006, 12, 2579–2586); these reagents nicely
complement the nucleophilic Ruppert–Prakash reagent (Me SiCF ), which we
3
3
mentioned in Review problem 4.5.
CF 3 CF 3
I I
O O
Me Me O
Togni reagent 1 Togni reagent 2
The Togni reagents, as these compounds have come to be known, trifluoromethylate
a variety of functional groups, including alcohols, phenols, thiols, -ketoesters and
-nitroesters:
OH OH OH
F C
CF 3 3 CF 3
Togni reagent 2
(a) +
Cl , RT
CH 2
2
Me Me Me
Me Me Me
Me Me Me
OH OH
Me Me
Togni reagent 2
(b)
DMF, − 60 °C,
18-crown-6
NaO HO
CF
3