Page 312 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 312
THE HALOGENS
292
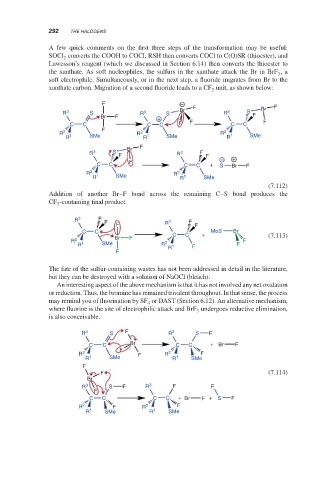
A few quick comments on the first three steps of the transformation may be useful:
SOCl converts the COOH to COCl, RSH then converts COCl to C(O)SR (thioester), and
2
Lawesson’s reagent (which we discussed in Section 6.14) then converts the thioester to
the xanthate. As soft nucleophiles, the sulfurs in the xanthate attack the Br in BrF ,a
3
soft electrophile. Simultaneously, or in the next step, a fluoride migrates from Br to the
xanthate carbon. Migration of a second fluoride leads to a CF unit, as shown below:
2
F − F F
R 3 S R 3 S Br R 3 S F Br
Br F +
C C C C F F C C F
F
R 2 1 SMe R 2 R 2 1 SMe
R R 1 SMe R
Br F
R 3 S R 3 F
F F − +
F
C C C C + S Br F
R 2 R 2
R 1 SMe R 1 SMe
(7.112)
Addition of another Br–F bond across the remaining C–S bond produces the
CF -containing final product.
3
R 3 F
F F R 3 F F
C C C C + MeS Br (7.113)
R 2 1 Br F 2 F
R SMe R 1 F
R F
F
The fate of the sulfur-containing wastes has not been addressed in detail in the literature,
but they can be destroyed with a solution of NaOCl (bleach).
An interesting aspect of the above mechanism is that it has not involved any net oxidation
or reduction. Thus, the bromine has remained trivalent throughout. In that sense, the process
may remind you of fluorination by SF or DAST (Section 6.12). An alternative mechanism,
4
where fluorine is the site of electrophilic attack and BrF undergoes reductive elimination,
3
is also conceivable.
R 3 S F R 3 S F
C C Br C C + Br F
F
R 2 F R 2 F
R 1 SMe R 1 SMe
F
F (7.114)
Br
R 3 S F R 3 F F
F
C C C C + Br F + S F
R 2 F R 2 F
R 1 SMe R 1 SMe