Page 314 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 314
THE HALOGENS
294
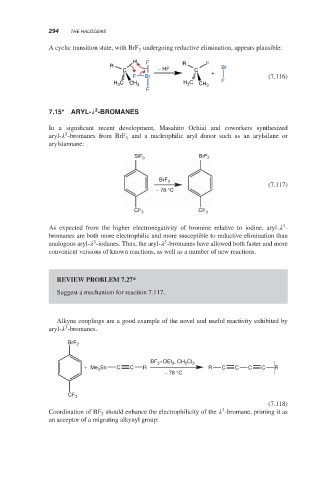
A cyclic transition state, with BrF undergoing reductive elimination, appears plausible:
3
H F F
R R Br
C − HF C +
F Br (7.116)
H 3 C CH 3 H 3 C CH 3 F
F
3
7.15* ARYL- -BROMANES
In a significant recent development, Masahito Ochiai and coworkers synthesized
3
aryl- -bromanes from BrF and a nucleophilic aryl donor such as an arylsilane or
3
arylstannane:
SiF 3 BrF 2
BrF 3
(7.117)
− 78 °C
CF 3 CF 3
3
As expected from the higher electronegativity of bromine relative to iodine, aryl- -
bromanes are both more electrophilic and more susceptible to reductive elimination than
3
3
analogous aryl- -iodanes. Thus, the aryl- -bromanes have allowed both faster and more
convenient versions of known reactions, as well as a number of new reactions.
REVIEW PROBLEM 7.27*
Suggest a mechanism for reaction 7.117.
Alkyne couplings are a good example of the novel and useful reactivity exhibited by
3
aryl- -bromanes.
BrF 2
BF 3 OEt 2 , CH 2 CI 2
+ Me Sn C C R R C C C C R
3
− 78 °C
CF 3
(7.118)
3
Coordination of BF should enhance the electrophilicity of the -bromane, priming it as
3
an acceptor of a migrating alkynyl group: