Page 318 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 318
THE HALOGENS
298
3
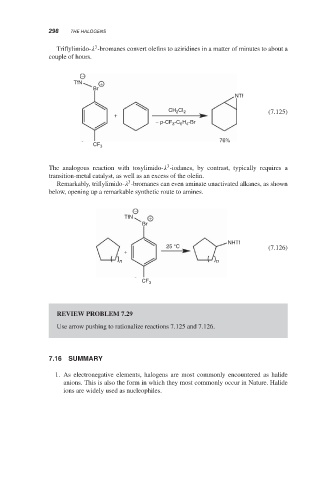
Triflylimido- -bromanes convert olefins to aziridines in a matter of minutes to about a
couple of hours.
−
TfN +
Br
NTf
CH 2 CI 2 (7.125)
+
-C H -Br
− p-CF 3 6 4
76%
CF 3
3
The analogous reaction with tosylimido- -iodanes, by contrast, typically requires a
transition-metal catalyst, as well as an excess of the olefin.
3
Remarkably, triflylimido- -bromanes can even aminate unactivated alkanes, as shown
below, opening up a remarkable synthetic route to amines.
−
TfN +
Br
NHTf
25 °C (7.126)
+
n n
CF 3
REVIEW PROBLEM 7.29
Use arrow pushing to rationalize reactions 7.125 and 7.126.
7.16 SUMMARY
1. As electronegative elements, halogens are most commonly encountered as halide
anions. This is also the form in which they most commonly occur in Nature. Halide
ions are widely used as nucleophiles.