Page 316 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 316
THE HALOGENS
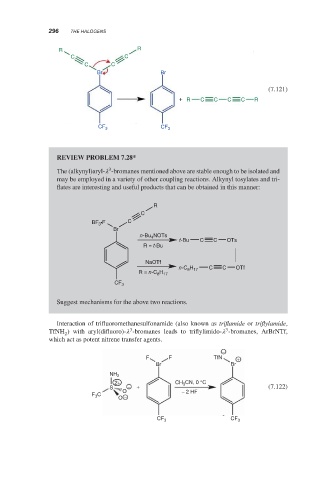
296
R R
C C
C C
Br Br
(7.121)
+ R C C C C R
CF 3 CF 3
REVIEW PROBLEM 7.28*
3
The (alkynyl)aryl- -bromanes mentioned above are stable enough to be isolated and
may be employed in a variety of other coupling reactions. Alkynyl tosylates and tri-
flates are interesting and useful products that can be obtained in this manner:
R
C
BF 3 •F C
Br
n-Bu NOTs
4
t-Bu C C OTs
R = t-Bu
NaOTf
n-C H C C OTf
8 17
H
R = n-C 8 17
CF 3
Suggest mechanisms for the above two reactions.
Interaction of trifluoromethanesulfonamide (also known as triflamide or triflylamide,
3
3
TfNH ) with aryl(difluoro)- -bromanes leads to triflylimido- -bromanes, ArBrNTf,
2
which act as potent nitrene transfer agents.
−
F F TfN +
Br Br
NH 2
2+ CH 3 CN, 0 °C
S − + (7.122)
O − 2 HF
F C
3
O −
CF 3 CF 3