Page 311 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 311
7.14 BROMINE TRIFLUORIDE 291
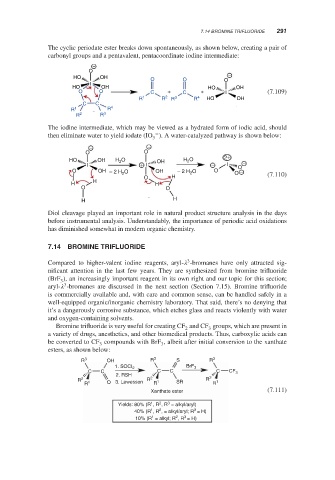
The cyclic periodate ester breaks down spontaneously, as shown below, creating a pair of
carbonyl groups and a pentavalent, pentacoordinate iodine intermediate:
−
O
HO OH −
O O O
I
HO OH HO OH
O O C + C + I (7.109)
R 1 R 2 R 3 R 4 HO OH
C C
R 1 R 4
R 2 R 3
The iodine intermediate, which may be viewed as a hydrated form of iodic acid, should
−
then eliminate water to yield iodate (IO ). A water-catalyzed pathway is shown below:
3
− −
O O
HO OH H O OH H 2 O 2+
2
I + I − I O −
O OH − 2 H 2 O OH − 2 H 2 O O O −
O H (7.110)
H
H H
O O
H H
Diol cleavage played an important role in natural product structure analysis in the days
before instrumental analysis. Understandably, the importance of periodic acid oxidations
has diminished somewhat in modern organic chemistry.
BROMINE TRIFLUORIDE
7.14
3
Compared to higher-valent iodine reagents, aryl- -bromanes have only attracted sig-
nificant attention in the last few years. They are synthesized from bromine trifluoride
(BrF ), an increasingly important reagent in its own right and our topic for this section;
3
3
aryl- -bromanes are discussed in the next section (Section 7.15). Bromine trifluoride
is commercially available and, with care and common sense, can be handled safely in a
well-equipped organic/inorganic chemistry laboratory. That said, there’s no denying that
it’s a dangerously corrosive substance, which etches glass and reacts violently with water
and oxygen-containing solvents.
Bromine trifluoride is very useful for creating CF and CF groups, which are present in
3
2
a variety of drugs, anesthetics, and other biomedical products. Thus, carboxylic acids can
be converted to CF compounds with BrF , albeit after initial conversion to the xanthate
3
3
esters, as shown below:
R 3 OH R 3 S R 3
1. SOCI 2 BrF 3
C C C C C CF 3
2. RSH
R 2 R 2 1 SR R 2 1
R 1 O 3. Lawesson R R
Xanthate ester (7.111)
3
2
1
Yields: 80% (R , R , R = alkyl/aryl)
3
2
1
40% (R , R , = alkyl/aryl; R = H)
1
2
3
10% (R = alkyl; R , R = H)