Page 309 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 309
5
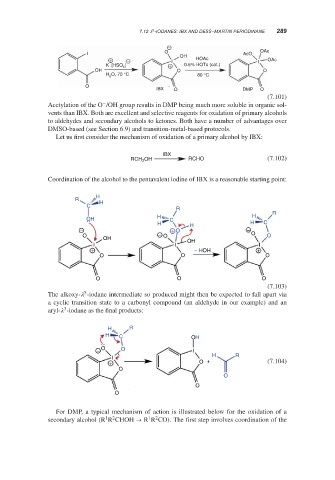
7.12 -IODANES: IBX AND DESS–MARTIN PERIODINANE 289
−
O OAc
I OH AcO
+ − I HOAc I OAc
K [HSO ] 5 + 0.5% HOTs (cat.)
OH O O
H O, 70 °C 80 °C
2
O
IBX O DMP O
(7.101)
−
Acetylation of the O /OH group results in DMP being much more soluble in organic sol-
vents than IBX. Both are excellent and selective reagents for oxidation of primary alcohols
to aldehydes and secondary alcohols to ketones. Both have a number of advantages over
DMSO-based (see Section 6.9) and transition-metal-based protocols.
Let us first consider the mechanism of oxidation of a primary alcohol by IBX:
IBX
RCH 2 OH RCHO (7.102)
Coordination of the alcohol to the pentavalent iodine of IBX is a reasonable starting point:
H
R
H
C
R
R
H
OH H C
H H H C
− + O −
O − O O O
OH OH
I I I I
+ − HOH +
O O O
O O O
(7.103)
5
The alkoxy- -iodane intermediate so produced might then be expected to fall apart via
a cyclic transition state to a carbonyl compound (an aldehyde in our example) and an
3
aryl- -iodane as the final products:
H R
H C OH
− O O I
H R
I
+ O + (7.104)
O
O
O
O
For DMP, a typical mechanism of action is illustrated below for the oxidation of a
1 2
1 2
secondary alcohol (R R CHOH → R R CO). The first step involves coordination of the