Page 310 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 310
THE HALOGENS
290
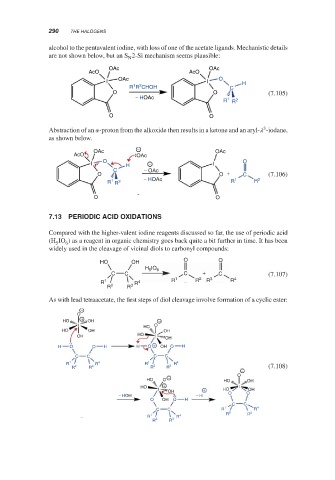
alcohol to the pentavalent iodine, with loss of one of the acetate ligands. Mechanistic details
are not shown below, but an S 2-Si mechanism seems plausible:
N
OAc OAc
AcO AcO
OAc O
I I H
1 2
R R CHOH C
O O (7.105)
− HOAc 1
R R 2
O O
3
Abstraction of an -proton from the alkoxide then results in a ketone and an aryl- -iodane,
as shown below.
−
OAc OAc
AcO OAc
O O
I H − I
C − OAc
O O + C (7.106)
1 − HOAc R 1 R 2
R R 2
O O
7.13 PERIODIC ACID OXIDATIONS
Compared with the higher-valent iodine reagents discussed so far, the use of periodic acid
(H IO ) as a reagent in organic chemistry goes back quite a bit further in time. It has been
5
6
widely used in the cleavage of vicinal diols to carbonyl compounds:
O O
HO OH
H 5 IO 6
C C C + C (7.107)
R 1 R 4 R 1 R 2 R 3 R 4
R 2 R 3
As with lead tetraacetate, the first steps of diol cleavage involve formation of a cyclic ester:
−
O
+
HO OH −
I HO O
HO OH OH
HO
OH I
OH
H O O H H O + OH O H
C C C C
R 1 R 4 R 1 R 4
R 2 R 3 R 2 R 3 (7.108)
−
O
−
HO O HO OH
HO + I
I + HO OH
OH O O
− HOH − H
O OH O H
C C
C C R 1 R 4
R 2 R 3
R 1 R 4
R 2 R 3