Page 304 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 304
THE HALOGENS
284
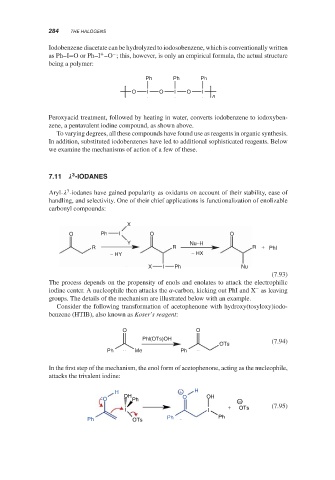
Iodobenzene diacetate can be hydrolyzed to iodosobenzene, which is conventionally written
+ −
as Ph–I=OorPh–I –O ; this, however, is only an empirical formula, the actual structure
being a polymer:
Ph Ph Ph
O I O I O I
n
Peroxyacid treatment, followed by heating in water, converts iodobenzene to iodoxyben-
zene, a pentavalent iodine compound, as shown above.
To varying degrees, all these compounds have found use as reagents in organic synthesis.
In addition, substituted iodobenzenes have led to additional sophisticated reagents. Below
we examine the mechanisms of action of a few of these.
3
7.11 -IODANES
3
Aryl- -iodanes have gained popularity as oxidants on account of their stability, ease of
handling, and selectivity. One of their chief applications is functionalization of enolizable
carbonyl compounds:
X
O Ph I O O
Y Nu–H
R R R + Phl
− HY − HX
X I Ph Nu
(7.93)
The process depends on the propensity of enols and enolates to attack the electrophilic
−
iodine center. A nucleophile then attacks the -carbon, kicking out PhI and X as leaving
groups. The details of the mechanism are illustrated below with an example.
Consider the following transformation of acetophenone with hydroxy(tosyloxy)iodo-
benzene (HTIB), also known as Koser’s reagent:
O O
Phl(OTs)OH (7.94)
OTs
Ph Me Ph
In the first step of the mechanism, the enol form of acetophenone, acting as the nucleophile,
attacks the trivalent iodine:
H + H
O OH Ph O OH −
+ OTs (7.95)
I I
Ph OTs Ph Ph