Page 303 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 303
7.10 AN INTRODUCTION TO HIGHER-VALENT ORGANOIODINE COMPOUNDS 283
Very often, the isocyanate is not isolated but is allowed to hydrolyze to the amine under the
reaction conditions:
O
O O
C H 2 O
N C RNH 2 + C (7.90)
N H
R OH O
R
7.10 AN INTRODUCTION TO HIGHER-VALENT ORGANOIODINE
COMPOUNDS
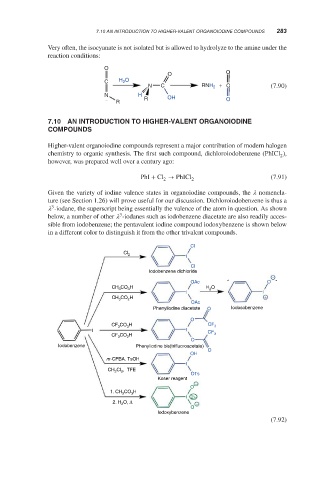
Higher-valent organoiodine compounds represent a major contribution of modern halogen
chemistry to organic synthesis. The first such compound, dichloroiodobenzene (PhICl ),
2
however, was prepared well over a century ago:
PhI + Cl → PhICl 2 (7.91)
2
Given the variety of iodine valence states in organoiodine compounds, the nomencla-
ture (see Section 1.26) will prove useful for our discussion. Dichloroiodobenzene is thus a
3
-iodane, the superscript being essentially the valence of the atom in question. As shown
3
below, a number of other -iodanes such as iodobenzene diacetate are also readily acces-
sible from iodobenzene; the pentavalent iodine compound iodoxybenzene is shown below
in a different color to distinguish it from the other trivalent compounds.
CI
Cl
2
I
CI
Iodobenzene dichloride
−
OAc “ O ”
CH CO H H O
3
3
2
I I
CH CO H +
2
3
OAc
Phenyliodine diacetate O Iodosobenzene
O
CF CO H CF
3 3 3
I I CF
CF CO H 3
3
2
O
Iodobenzene Phenyliodine bis(trifluoroacetate)
O
OH
m-CPBA, TsOH
I
CH Cl , TFE
2
2
OTs
Koser reagent
−
O
1. CH CO H
3
3
I 2+
2. H O, Δ
2 −
O
Iodoxybenzene
(7.92)