Page 301 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 301
7.9 HALOGENS IN ORGANIC SYNTHESIS: SOME CLASSICAL REACTIONS 281
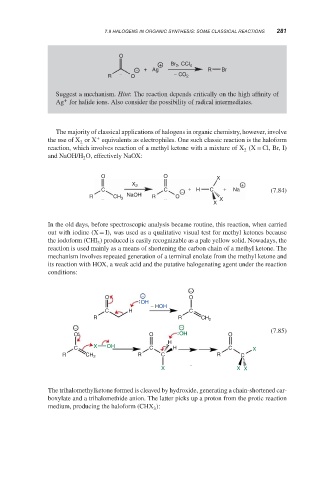
O
+ Br 2 , CCl 4
− + Ag R Br
R O − CO 2
Suggest a mechanism. Hint: The reaction depends critically on the high affinity of
+
Ag for halide ions. Also consider the possibility of radical intermediates.
The majority of classical applications of halogens in organic chemistry, however, involve
+
the use of X or X equivalents as electrophiles. One such classic reaction is the haloform
2
reaction, which involves reaction of a methyl ketone with a mixture of X (X = Cl, Br, I)
2
and NaOH/H O, effectively NaOX:
2
O O X
X 2 +
C C − + H C + Na (7.84)
R CH 3 NaOH R O
X
X
In the old days, before spectroscopic analysis became routine, this reaction, when carried
out with iodine (X = I), was used as a qualitative visual test for methyl ketones because
the iodoform (CHI ) produced is easily recognizable as a pale yellow solid. Nowadays, the
3
reaction is used mainly as a means of shortening the carbon chain of a methyl ketone. The
mechanism involves repeated generation of a terminal enolate from the methyl ketone and
its reaction with HOX, a weak acid and the putative halogenating agent under the reaction
conditions:
−
−
O O
OH
− HOH
C H C
R R CH 2
− −
O O OH O (7.85)
H
C X OH C H C X
R CH 2 R C R C
X X X
The trihalomethylketone formed is cleaved by hydroxide, generating a chain-shortened car-
boxylate and a trihalomethide anion. The latter picks up a proton from the protic reaction
medium, producing the haloform (CHX ):
3