Page 300 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 300
THE HALOGENS
280
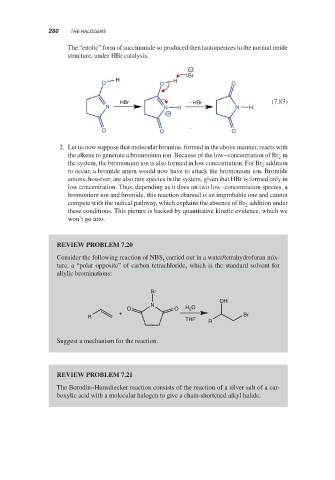
The “enolic” form of succinimide so produced then tautomerizes to the normal imide
structure, under HBr catalysis.
−
Br
H H
O O O
HBr − HBr (7.83)
N N H N H
+
O O O
2. Let us now suppose that molecular bromine, formed in the above manner, reacts with
the alkene to generate a bromonium ion. Because of the low–concentration of Br in
2
the system, the bromonium ion is also formed in low concentration. For Br addition
2
to occur, a bromide anion would now have to attack the bromonium ion. Bromide
anions, however, are also rare species in the system, given that HBr is formed only in
low concentration. Thus, depending as it does on two low–concentration species, a
bromonium ion and bromide, this reaction channel is an improbable one and cannot
compete with the radical pathway, which explains the absence of Br addition under
2
these conditions. This picture is backed by quantitative kinetic evidence, which we
won’t go into.
REVIEW PROBLEM 7.20
Consider the following reaction of NBS, carried out in a water/tetrahydrofuran mix-
ture, a “polar opposite” of carbon tetrachloride, which is the standard solvent for
allylic brominations:
Br
OH
N
O O H 2 O
+
R Br
THF R
Suggest a mechanism for the reaction.
REVIEW PROBLEM 7.21
The Borodin–Hunsdiecker reaction consists of the reaction of a silver salt of a car-
boxylic acid with a molecular halogen to give a chain-shortened alkyl halide.