Page 317 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 317
3
7.15 ARYL- -BROMANES 297
Interestingly, an analogous synthesis cannot be accomplished with p-toluenesulfonamide
(tosylamide, TsNH ), indicating the need for a group that is more electron withdrawing
2
than Ts.
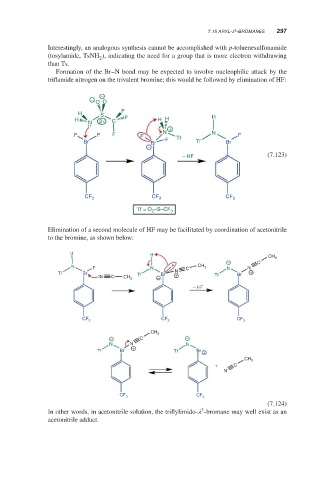
Formation of the Br–N bond may be expected to involve nucleophilic attack by the
triflamide nitrogen on the trivalent bromine; this would be followed by elimination of HF:
−
−
O O
F
H S
H 2+ C F H H H
N
+
N N
F F F F Tf F
Br Br F Tf Br
−
− HF (7.123)
CF 3 CF 3 CF 3
Tf = O 2 –S–CF 3
Elimination of a second molecule of HF may be facilitated by coordination of acetonitrile
to the bromine, as shown below:
H
H CH
3
− C
N F N F C CH 3 N N
Tf Br Tf Br N Br +
N C CH 3 − + Tf
− HF
CF CF CF 3
3 3
CH
3
− C −
N N N
Tf Br + Tf Br +
CH 3
+ C
N
CF
CF 3 3
(7.124)
3
In other words, in acetonitrile solution, the triflylimido- -bromane may well exist as an
acetonitrile adduct.