Page 282 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 282
THE HALOGENS
262
The process may be viewed as a chain reaction, where the first step is the radical initiation,
the second and the third steps are propagation, and the last step is radical termination.
Despite its name, hypofluorous acid is not significantly acidic and does not ionize in
water. It is, however, highly reactive and even oxidizes water to H O :
2
2
HOF + H O → H O + HF (7.26)
2
2
2
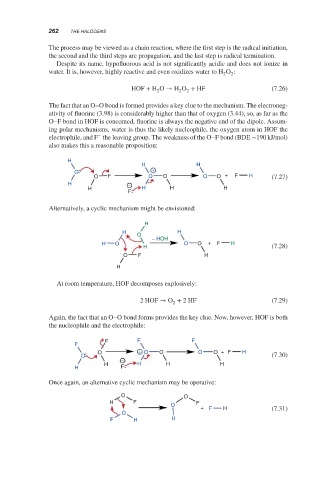
The fact that an O–O bond is formed provides a key clue to the mechanism. The electroneg-
ativity of fluorine (3.98) is considerably higher than that of oxygen (3.44), so, as far as the
O–F bond in HOF is concerned, fluorine is always the negative end of the dipole. Assum-
ing polar mechanisms, water is thus the likely nucleophile, the oxygen atom in HOF the
−
electrophile, and F the leaving group. The weakness of the O–F bond (BDE ∼190 kJ/mol)
also makes this a reasonable proposition:
H
H H
O +
O F O O O O + F H (7.27)
H −
H H H H
F
Alternatively, a cyclic mechanism might be envisioned:
H
H H
O
− HOH
H O O O + F H
H (7.28)
O F H
H
At room temperature, HOF decomposes explosively:
2 HOF → O + 2 HF (7.29)
2
Again, the fact that an O–O bond forms provides the key clue. Now, however, HOF is both
the nucleophile and the electrophile:
F F F
F
O + O O O O + F H
O (7.30)
−
H H H H
H F
Once again, an alternative cyclic mechanism may be operative:
O O
H F F
O
+ F H (7.31)
O
F H H