Page 333 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 333
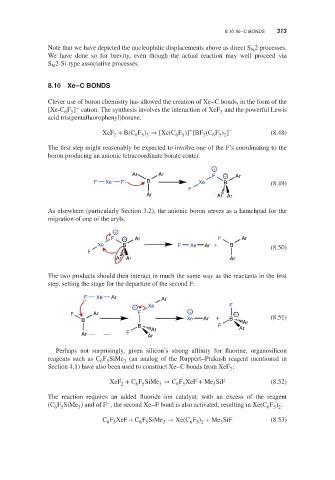
8.10 Xe–C BONDS 313
Note that we have depicted the nucleophilic displacements above as direct S 2 processes.
N
We have done so for brevity, even though the actual reaction may well proceed via
S 2-Si-type associative processes.
N
8.10 Xe–C BONDS
Clever use of boron chemistry has allowed the creation of Xe–C bonds, in the form of the
+
[Xe-C F ] cation. The synthesis involves the interaction of XeF and the powerful Lewis
2
6 5
acid tris(pentafluorophenyl)borane.
+
XeF + B(C F ) → [Xe(C F )] [BF (C F ) ] − (8.48)
6 5
2
6 5 3
6 5 2
2
The first step might reasonably be expected to involve one of the F’s coordinating to the
boron producing an anionic tetracoordinate borate center.
+
Ar Ar F − Ar
F Xe F B Xe B (8.49)
F
Ar Ar Ar
As elsewhere (particularly Section 3.2), the anionic boron serves as a launchpad for the
migration of one of the aryls.
+
F − Ar F Ar
Xe B F Xe Ar + B (8.50)
F
Ar Ar Ar
The two products should then interact in much the same way as the reactants in the first
step, setting the stage for the departure of the second F:
F Xe Ar Ar
+ Xe F
F Ar F + + −
B Xe Ar B Ar (8.51)
B Ar F Ar
Ar F Ar
Perhaps not surprisingly, given silicon’s strong affinity for fluorine, organosilicon
reagents such as C F SiMe (an analog of the Ruppert–Prakash reagent mentioned in
3
6 5
Section 4.1) have also been used to construct Xe–C bonds from XeF :
2
XeF + C F SiMe → C F XeF + Me SiF (8.52)
3
6 5
6 5
2
3
The reaction requires an added fluoride ion catalyst; with an excess of the reagent
−
(C F SiMe ) and of F , the second Xe–F bond is also activated, resulting in Xe(C F ) :
6 5 2
3
6 5
C F XeF + C F SiMe → Xe(C F ) + Me SiF (8.53)
6 5
6 5
6 5 2
3
3