Page 328 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 328
THE NOBLE GASES
308
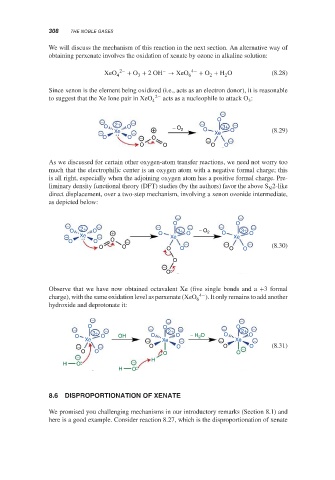
We will discuss the mechanism of this reaction in the next section. An alternative way of
obtaining perxenate involves the oxidation of xenate by ozone in alkaline solution:
−
XeO 4 2− + O + 2OH → XeO 6 4− + O + H O (8.28)
3
2
2
Since xenon is the element being oxidized (i.e., acts as an electron donor), it is reasonable
to suggest that the Xe lone pair in XeO 4 2− acts as a nucleophile to attack O :
3
−
− − − O
O 2+ O − O 3+ −
− Xe − + 2 O Xe O (8.29)
O O − O − −
O O O O
As we discussed for certain other oxygen-atom transfer reactions, we need not worry too
much that the electrophilic center is an oxygen atom with a negative formal charge; this
is all right, especially when the adjoining oxygen atom has a positive formal charge. Pre-
liminary density functional theory (DFT) studies (by the authors) favor the above S 2-like
N
direct displacement, over a two-step mechanism, involving a xenon ozonide intermediate,
as depicted below:
− −
− 2+ − − O − − O −
O O + O 3+ O − O 2 O 3+ O
− Xe − Xe Xe
O O O − − − −
O O O O O O (8.30)
O
−
O
Observe that we have now obtained octavalent Xe (five single bonds and a +3formal
charge), with the same oxidation level as perxenate (XeO 6 4− ). It only remains to add another
hydroxide and deprotonate it:
− − −
O O − O
− 3+ − − 2+ − 2+ −
O O OH O O − H O O O
2
Xe − Xe − − Xe −
− − O O O − O (8.31)
O O O O
− H
H O −
H O
8.6 DISPROPORTIONATION OF XENATE
We promised you challenging mechanisms in our introductory remarks (Section 8.1) and
here is a good example. Consider reaction 8.27, which is the disproportionation of xenate