Page 327 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 327
8.5 XENATE AND PERXENATE 307
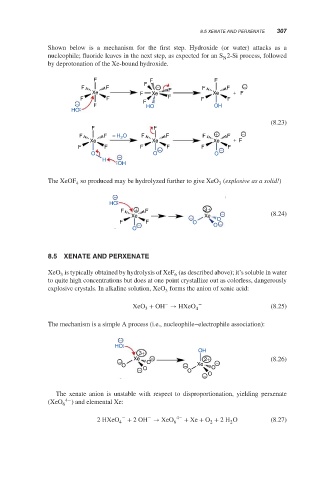
Shown below is a mechanism for the first step. Hydroxide (or water) attacks as a
nucleophile; fluoride leaves in the next step, as expected for an S 2-Si process, followed
N
by deprotonation of the Xe-bound hydroxide.
F F F
F
F F − F F −
Xe F Xe F Xe + F
F F F F F
− F F HO OH
HO
(8.23)
F F
F F − H 2 O F F F + F −
Xe Xe Xe + F
F F F − F F − F
O O O
H −
OH
The XeOF so produced may be hydrolyzed further to give XeO (explosive as a solid!)
4
3
−
HO
F + F 3+
Xe − Xe − (8.24)
O
F − F O O −
O
8.5 XENATE AND PERXENATE
XeO is typically obtained by hydrolysis of XeF (as described above); it’s soluble in water
6
3
to quite high concentrations but does at one point crystallize out as colorless, dangerously
explosive crystals. In alkaline solution, XeO forms the anion of xenic acid:
3
− −
XeO + OH → HXeO 4 (8.25)
3
The mechanism is a simple A process (i.e., nucleophile–electrophile association):
−
HO
3+ OH
− Xe O − 2+ − (8.26)
O − Xe O
− O O
− O
The xenate anion is unstable with respect to disproportionation, yielding perxenate
4−
(XeO 6 ) and elemental Xe:
− − 4−
2 HXeO 4 + 2OH → XeO 6 + Xe + O + 2H O (8.27)
2
2