Page 329 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 329
8.6 DISPROPORTIONATION OF XENATE 309
to perxenate and elemental xenon in alkaline solution; elemental oxygen is also produced
at the same time:
− − 4−
2 HXeO + 2OH → XeO + Xe + O + 2H O (8.32)
4 6 2 2
By now, we trust you have exercised your arrow-pushing skills a good deal. Nevertheless,
as a mechanistic exercise, this reaction may leave you perplexed. How, for example, would
one go about stripping all those oxygens from xenate to produce elemental xenon? Before
thinking through that question, it might help to focus on the slightly less daunting half of
the problem: how does xenate get oxidized to perxenate?
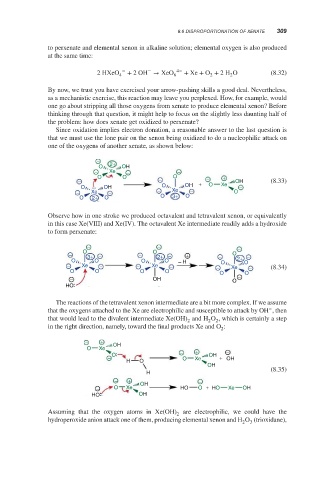
Since oxidation implies electron donation, a reasonable answer to the last question is
that we must use the lone pair on the xenon being oxidized to do a nucleophilic attack on
one of the oxygens of another xenate, as shown below:
−
O 2+ OH
− Xe − −
O O O +
− − + − OH (8.33)
O OH O OH O Xe −
− Xe − − Xe − O
O 2+ O O 3+ O
Observe how in one stroke we produced octavalent and tetravalent xenon, or equivalently
in this case Xe(VIII) and Xe(IV). The octavalent Xe intermediate readily adds a hydroxide
to form perxenate:
− − −
− O 3+ − − O 2+ − + − O −
O O O O − H O 2+ O
− Xe − − Xe − − Xe − (8.34)
O O O O O O
− OH O −
HO
The reactions of the tetravalent xenon intermediate are a bit more complex. If we assume
−
that the oxygens attached to the Xe are electrophilic and susceptible to attack by OH , then
that would lead to the divalent intermediate Xe(OH) and H O , which is certainly a step
2
2
2
in the right direction, namely, toward the final products Xe and O :
2
− + OH
O Xe − + −
− O O Xe OH + OH
H O
OH
(8.35)
H
− + −
− O Xe OH HO O + HO Xe OH
HO OH
Assuming that the oxygen atoms in Xe(OH) are electrophilic, we could have the
2
hydroperoxide anion attack one of them, producing elemental xenon and H O (trioxidane),
2
3