Page 331 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 331
8.8 OTHER COMPOUNDS CONTAINING Xe–O BONDS 311
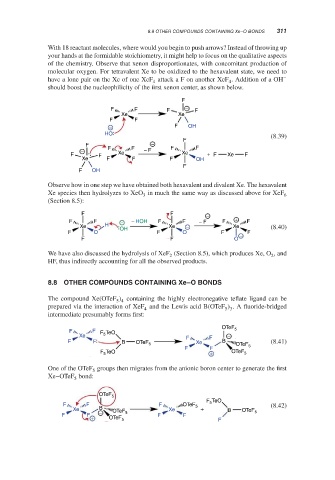
With 18 reactant molecules, where would you begin to push arrows? Instead of throwing up
your hands at the formidable stoichiometry, it might help to focus on the qualitative aspects
of the chemistry. Observe that xenon disproportionates, with concomitant production of
molecular oxygen. For tetravalent Xe to be oxidized to the hexavalent state, we need to
have a lone pair on the Xe of one XeF attackaFon another XeF . Addition of a OH −
4 4
should boost the nucleophilicity of the first xenon center, as shown below.
F
F F F − F
Xe Xe
F F
− F OH
HO (8.39)
F
F −
− F F − F F F
F F Xe Xe + F Xe F
Xe F F F OH
F
F OH
Observe how in one step we have obtained both hexavalent and divalent Xe. The hexavalent
Xe species then hydrolyzes to XeO in much the same way as discussed above for XeF
3 6
(Section 8.5):
F F −
F F − − HOH F F − F F + F
Xe H OH Xe − Xe (8.40)
F O F O F − F
F F O
We have also discussed the hydrolysis of XeF (Section 8.5), which produces Xe, O , and
2
2
HF, thus indirectly accounting for all the observed products.
8.8 OTHER COMPOUNDS CONTAINING Xe–O BONDS
The compound Xe(OTeF ) containing the highly electronegative teflate ligand can be
5 4
prepared via the interaction of XeF and the Lewis acid B(OTeF ) . A fluoride-bridged
5 3
4
intermediate presumably forms first:
OTeF 5
F F TeO
Xe F 5 F F −
F F B OTeF 5 Xe B OTeF (8.41)
F F 5
TeO OTeF
F 5 + 5
One of the OTeF groups then migrates from the anionic boron center to generate the first
5
Xe–OTeF bond:
5
OTeF 5
F TeO
5
F F F OTeF 5 (8.42)
Xe B OTeF Xe + B OTeF 5
F F − 5 F F
+ OTeF 5 F