Page 142 - Assurance of Sterility for Sensitive Combination Products and Materials
P. 142
124 Assurance of sterility for sensitive combination products and materials
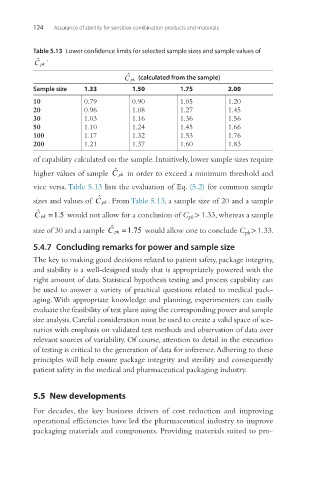
Table 5.13 Lower confidence limits for selected sample sizes and sample values of
.
C pk
C pk (calculated from the sample)
Sample size 1.33 1.50 1.75 2.00
10 0.79 0.90 1.05 1.20
20 0.96 1.08 1.27 1.45
30 1.03 1.16 1.36 1.56
50 1.10 1.24 1.45 1.66
100 1.17 1.32 1.53 1.76
200 1.21 1.37 1.60 1.83
of capability calculated on the sample. Intuitively, lower sample sizes require
higher values of sample C pk in order to exceed a minimum threshold and
vice versa. Table 5.13 lists the evaluation of Eq. (5.2) for common sample
sizes and values of C pk . From Table 5.13, a sample size of 20 and a sample
C pk =15 would not allow for a conclusion of C pk > 1.33, whereas a sample
.
size of 30 and a sample C pk =175 would allow one to conclude C pk > 1.33.
.
5.4.7 Concluding remarks for power and sample size
The key to making good decisions related to patient safety, package integrity,
and stability is a well-designed study that is appropriately powered with the
right amount of data. Statistical hypothesis testing and process capability can
be used to answer a variety of practical questions related to medical pack-
aging. With appropriate knowledge and planning, experimenters can easily
evaluate the feasibility of test plans using the corresponding power and sample
size analysis. Careful consideration must be used to create a valid space of sce-
narios with emphasis on validated test methods and observation of data over
relevant sources of variability. Of course, attention to detail in the execution
of testing is critical to the generation of data for inference. Adhering to these
principles will help ensure package integrity and sterility and consequently
patient safety in the medical and pharmaceutical packaging industry.
5.5 New developments
For decades, the key business drivers of cost reduction and improving
operational efficiencies have led the pharmaceutical industry to improve
packaging materials and components. Providing materials suited to pro-