Page 306 - Battery Reference Book
P. 306
Calcium anode-based thermal batteries 27/3
7.6 Calcium anode-based thermal Much of the effort associated with thermal battery
batteries design involves determining the amount of pyrotechnic
heat to give acceptable performance over the necessary
27.1.1 Calcium-calcium chromate thermal temperature range. Many of the required temperature
batteries extremes range from -55 to +75T for initial ambi-
ent. This 130°C difference is well within the opera-
The overall reaction for the calcium-calcium chromate tional limits of 352"C, the melting point of lithium
system is dependent on the discharge parameters. A chloride-potassium chloride eutectic, and 6013"C, the
postulated cell reaction is: approximate temperature of thermal runaway. How-
3Ca + 2CaCr04 -t 6LiCI ever, as discussed earlier for calcium-calcium chro-
mate, a temperature of at least 485°C is necessary for
+ 3CaC12 + Cr203.2Ca0 + 3Lizo (27.1)
minimal performance at moderate drain ( >50mA/cm2)
although the exact composition of the mixed oxide has due to freeze-out of double salt (KCl.CaC12) below
not been determined. It is possible that lithium formed that temperature. Thus, it is seen that the working tem-
by reaction of calcium with lithium chloride electrolyte perature range is reduced to 115°C whereas military
enters into the electrochemical reaction. extremes exceed this by as much as 15°C.
Side-reactions occur between calcium and LiC1-
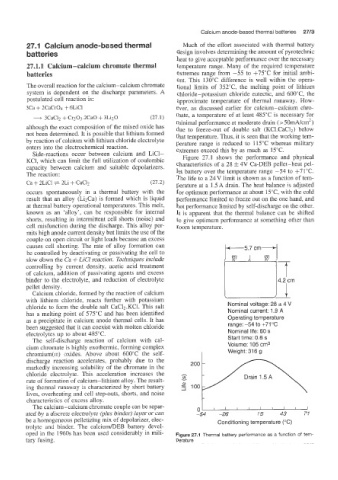
Figure 27.1 shows the performance and physical
KC1, which can limit the full utilization of coulombic characteristics of a 28 f 4V ea-DEB pellet-heat pel-
capacity between calcium and suitable depolarizers.
let battery over the temperature range -54 to +71"C.
The reaction: The life to a 24 V limit is shown as a function of tem-
Ca + 2LiCI + 2Li + CaC12 (27.2) perature at a 1 .5 A drain. The heat balance is adjusted
occurs spontaneously in a thermal battery with the for optimum performance at about 15T, with the cold
result that an alloy (Li2Ca) is formed which is liquid performance limited to freeze out on the one hand, and
at thermal battery operational temperatures. This melt, hot performance limited by self-discharge on the other.
known as an 'alloy', can be responsible for internal It is apparent that the thermal balance can be shifted
shorts, resulting in intermittent cell shorts (noise) and to give optimum performance at something other than
cell misfunction during the discharge. This alloy per- room temperature.
mits high anode current density but limits the use of the
couple on open circuit or light loads because an excess
causes cell shorting. The rate of alloy formation can -5.7 cm-
be controlled by deactivating or passivating the cell to PA w
slow down the Ca + LiCl reaction. Techniques include
controlling by current density, acetic acid treatment t
of calcium, addition of passivating agents and excess
binder to the electrolyte, and reduction of electrolyte 4.2 cm
pellet density.
Calcium chloride, formed by the reaction of calcium
with lithium chloride, reacts further with potassium
chloride to form the double salt CaCl2.KC1. This salt Nominal voltage: 28 2 4 V
has a melting point of 575°C and has been identified Nominal current: 1.9 A
as a precipitate in calcium anode thermal cells. It has Operating temperature
been suggested that it can coexist with molten chloride range: -54 to +71 "C
electrolytes up to about 485°C. Nominal life: 60 s
The self-discharge reaction of calcium with cal- Start time: 0.6 s
cium chromate is highly exothermic, forming complex Volume: 105 crn3
Weight: 316 g
chromhm(m) oxides. Above about 600°C the self- 200 1
discharge reaction accelerates, probably due to the
markedly increasing solubility of the chromate in the
chloride electrolyte. This acceleration increases the
rate of fornation of calcium-lithium alloy. The result-
ing thermal runaway Is characterized by short battery
lives, overheating and cell step-outs, shorts, and noise
characteristics of excess alloy.
The calcium-calcium chromate couple can be separ-
ated by a discrete electrolyte (plus binder) layer or can 0 -26 15 43 71
-54
be a homogeneous pelletizing mix of depolarizer, elec- Conditioning temperature ("C)
trolyte and binder. The calciumlDEB battery devel-
oped in the 1960s has been used considerably in mili- Figure 27.1 Thermal battery performance as a function of tem-
tary fusing. perature