Page 57 - Battery Reference Book
P. 57
1/42 Introduction to battery technology
96V
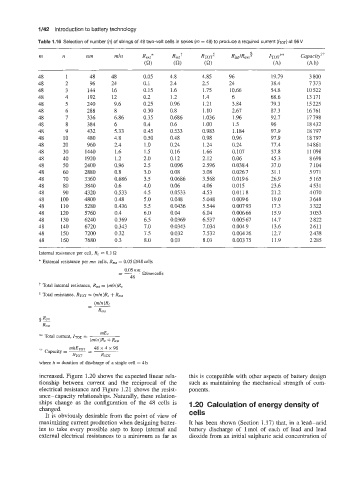
Table 1.16 Selection of number (n) of strings of 48 two-volt cells in series (rn = 48) to produce a required current (IToT)
at
rn n nrn mln Rex,* Riati RTOT* RintlRext’ ITOT** Capacity?+
(Q2) (Q) (a) (A) (A h)
48 1 48 48 0.05 4.8 4.85 96 19.79 3 800
48 2 96 24 0.1 2.4 2.5 24 38.4 7 313
48 3 144 16 0.15 1.6 1.75 10.66 54.8 10 522
48 4 192 12 0.2 1.2 1.4 6 68.6 13 171
48 5 240 9.6 0.25 0.96 1.21 3.84 79.3 15 225
48 6 288 8 0.30 0.8 1.10 2.67 87.3 16761
48 7 336 6.86 0.35 0.686 1.036 1.96 92.7 17 798
48 8 3 84 6 0.4 0.6 1 .oo 1.5 96 18432
48 9 432 5.33 0.45 0.533 0.983 1.184 97.9 18 797
48 10 480 4.8 0.50 0.48 0.98 0.96 97.9 18 797
48 20 960 2.4 1.0 0.24 1.24 0.24 77.4 14 861
48 30 1440 1.6 1.5 0.16 1.66 0.107 57.8 11 098
48 40 1920 1.2 2.0 0.12 2.12 0.06 45.3 8 698
48 50 2400 0.96 2.5 0.096 2.596 0.038 4 37.0 7 104
48 60 2880 0.8 3.0 0.08 3.08 0.026 7 31.1 5 971
48 70 3360 0.686 3.5 0.0686 3.568 0.0196 26.9 5 165
48 80 3840 0.6 4.0 0.06 4.06 0.015 23.6 4531
48 90 4320 0.533 4.5 0.0533 4.53 0.011 8 21.2 4 070
48 100 4800 0.48 5.0 0.048 5.048 0.009 6 19.0 3 648
48 110 5280 0.436 5.5 0.0436 5.544 0.007 93 17.3 3 322
48 120 5760 0.4 6.0 0.04 6.04 0.006 66 15.9 3 053
48 130 6240 0.369 6.5 0.0369 6.537 0.005 67 14.7 2 822
48 140 6720 0.343 7.0 0.0343 7.034 0.004 9 13.6 2611
48 150 7200 0.32 7.5 0.032 7.532 0.004 26 12.7 2 438
48 160 7680 0.3 8.0 0.03 8.03 0.003 75 11.9 2 285
Internal resistance per cell, R, = 0.1 $2
* External resistance per mn cells, ReXt = 0.05 Q/48 cells
0.05 nm
=- Q/nm cells
48
Total internal resistance, Rint = (m/n)R,
Total resistance, RT~T = (rn/n)R, +Re,,
(mln )Rc
-~
-
Rat
DR”
Rat
mEc
=
** Total current, I ~ T
(m/n )Rc + Rex,
where h = duration of discharge of a single cell = 4 h
increased. Figure 1.20 shows the expected linear rela- this is compatible with other aspects of battery design
tionship between current and the reciprocal of the such as maintaining the mechanical strength of com-
electrical resistance and Figure 1.21 shows the resist- ponents.
ance-capacity relationships. Naturally, these relation-
ships change as the configuration of the 48 cells is 1.20 Calculation of energy density of
changed.
It is obviously desirable from the point of view of cells
maximizing current production when designing batter- It has been shown (Section 1.17) that, in a lead-acid
ies to take every possible step to keep internal and battery discharge of lmol of each of lead and lead
external electrical resistances to a minimum as far as dioxide from an initial sulphuric acid concentration of