Page 62 - Battery Reference Book
P. 62
Heating effects in batteries 6/47
Capacity 0.03
- 30
- 20
- 26 I
- 24 1.6-
- 22
-
W h/kg - 1.5 - 0.02 5
X 20 2
- 18 5 - W
- 16 2 - C
> 1.4-
m
c
- 14 'i W ._
m
E
- 12 g m -
-
- 10 P 1.3- 0.01 6
?
-8 -0
5 1.2-
-6
-4 -
-2 1.1
L-- -0
0 4 8 12 16 20 24 28 1 .o I I I I I
0 100 200 300 400 500 600
Discharge Tim@ (min)
Discharge current (A)
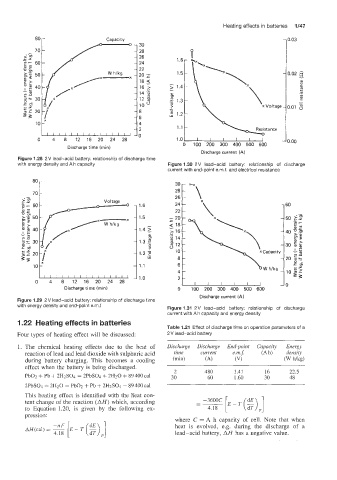
Figure 1.28 2V lead-acid battery: relationship of discharge time
with energy density and Ah capacity Figure 1.30 2V lead-acid battery: relationship of discharge
current with end-Doint e.m.f. and electrical resistance
1.6
1.5
1.4
W m
1.3 2 ta \ 130
?
-- w XCapacity
1.2 F
1.1
0 4 8 12 16 20 24 28 1.0 JO
Discharge time (mid 0 100 200 300 400 500 600
Discharge current (A)
Figure 1.29 2V lead-acid battery: relationship of discharge time
with energy density and end-point e.m.f Figure 1.31 2V lead-acid battery: relationship of discharge
current with Ah capacity and energy density
1.22 Heating effects in batteries
Table 1.21 Effect of discharge time on operation parameters of a
Four types of heating effect will be discussed: 2 V lead-acid battery
1. The chemical heating effects due to the heat of Discharge Discharge End-point Capacity Energy
density
reaction of lead and lead dioxide with sulphuric acid time current emf (Ah) (W m)
during battery charging. This becomes a cooling (min) (A) (VI
effect when the battery is being discharged. ~~
2 480 1.41 16 22.5
PbOz + Pb + 2HzSO4 = 2PbSO4 + 2H20 + 89 400 cal 30 60 1.60 30 48
2PbS04 + 2H20 = PbOz + Pb + 2HzS04 - 89 400 cal
This heating effect is identified with the lieat con-
tent change of the reaction (AH) which, according - -3600C
-
to Equation 1.20, is given by the following ex- 4.18
pression:
where C = A h capacity of cell. Note that when
heat is evolved, e.g. during the discharge of a
lead-acid battery, AH has a negative value.