Page 190 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 190
166 7 Synergies of Chemistry and Biochemistry for the Production of -Amino Acids
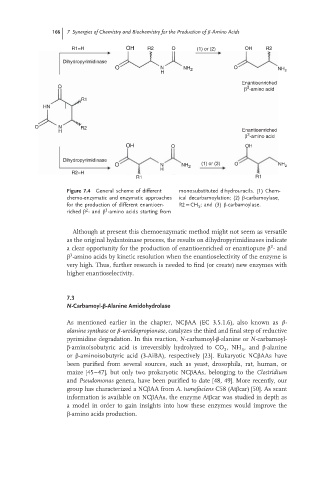
R1=H OH R2 O (1) or (2) OH R2
Dihydropyrimidinase
O N NH 2 O NH 2
H
Enantioenriched
O 3
β -amino acid
R1
HN
O N R2
H Enantioenriched
3
β -amino acid
OH O OH
Dihydropyrimidinase
O N NH 2 (1) or (3) O NH 2
H
R2=H
R1 R1
Figure 7.4 General scheme of different monosubstituted dihydrouracils. (1) Chem-
chemo-enzymatic and enzymatic approaches ical decarbamoylation; (2) β-carbamoylase,
for the production of different enantioen- R2 = CH ;and (3) β-carbamoylase.
3
2
3
riched β -and β -amino acids starting from
Although at present this chemoenzymatic method might not seem as versatile
as the original hydantoinase process, the results on dihydropyrimidinases indicate
2
a clear opportunity for the production of enantioenriched or enantiopure β -and
3
β -amino acids by kinetic resolution when the enantioselectivity of the enzyme is
very high. Thus, further research is needed to find (or create) new enzymes with
higher enantioselectivity.
7.3
N-Carbamoyl- -Alanine Amidohydrolase
As mentioned earlier in the chapter, NCβAA (EC 3.5.1.6), also known as -
alanine synthase or -ureidopropionase, catalyzes the third and final step of reductive
pyrimidine degradation. In this reaction, N-carbamoyl-β-alanine or N-carbamoyl-
β-aminoisobutyric acid is irreversibly hydrolyzed to CO ,NH ,and β-alanine
3
2
or β-aminoisobutyric acid (3-AiBA), respectively [23]. Eukaryotic NCβAAs have
been purified from several sources, such as yeast, drosophila, rat, human, or
maize [45–47], but only two prokaryotic NCβAAs, belonging to the Clostridium
and Pseudomonas genera, have been purified to date [48, 49]. More recently, our
group has characterized a NCβAA from A. tumefaciens C58 (Atβcar) [50]. As scant
information is available on NCβAAs, the enzyme Atβcar was studied in depth as
a model in order to gain insights into how these enzymes would improve the
β-amino acids production.