Page 196 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 196
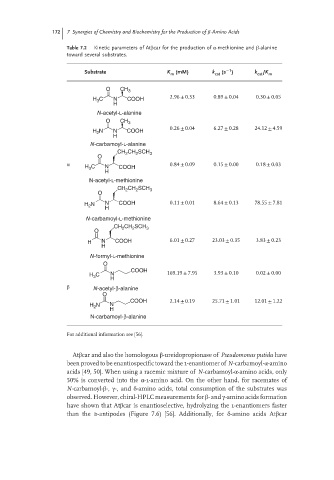
172 7 Synergies of Chemistry and Biochemistry for the Production of -Amino Acids
Table 7.2 Kinetic parameters of Atβcar for the production of α-methionine and β-alanine
toward several substrates.
−1
Substrate K m (mM) k cat (s ) k cat /K m
O CH 3
H C N COOH 2.96 ± 0.33 0.89 ± 0.04 0.30 ± 0.05
3
H
N-acetyl-L-alanine
O CH 3
0.26 ± 0.04 6.27 ± 0.28 24.12 ± 4.59
H N N COOH
2
H
N-carbamoyl-L-alanine
CH 2 CH 2 SCH 3
O
α 0.84 ± 0.09 0.15 ± 0.00 0.18 ± 0.03
H 3 C N COOH
H
N-acetyl-L-methionine
CH 2 CH 2 SCH 3
O
H N N COOH 0.11 ± 0.01 8.64 ± 0.13 78.55 ± 7.81
2
H
N-carbamoyl-L-methionine
CH CH SCH 3
2
2
O
H N COOH 6.01 ± 0.27 23.03 ± 0.35 3.83 ± 0.23
H
N-formyl-L-methionine
O
COOH
H C N 169.19 ± 7.95 3.93 ± 0.10 0.02 ± 0.00
3
H
β N-acetyl-β-alanine
O
COOH 2.14 ± 0.19 25.71 ± 1.01 12.01 ± 1.22
H 2 N N
H
N-carbamoyl-β-alanine
For additional information see [56].
Atβcar and also the homologous β-ureidopropionase of Pseudomonas putida have
been proved to be enantiospecific toward the l-enantiomer of N-carbamoyl-α-amino
acids [49, 50]. When using a racemic mixture of N-carbamoyl-α-amino acids, only
50% is converted into the α-l-amino acid. On the other hand, for racemates of
N-carbamoyl-β-, γ-, and δ-amino acids, total consumption of the substrates was
observed. However, chiral-HPLC measurements for β-and γ-amino acids formation
have shown that Atβcar is enantioselective, hydrolyzing the l-enantiomers faster
than the d-antipodes (Figure 7.6) [56]. Additionally, for δ-amino acids Atβcar