Page 197 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 197
7.4 Bienzymatic System for β-Amino Acid Production 173
120 120
100 100
Conversion (%) 80 Conversion (%) 80
60
60
40
20 40
20
0 0
0 2 4 6 8 10 12 0 2 4 6 8 10 12
(a) Time (h) (b) Time (h)
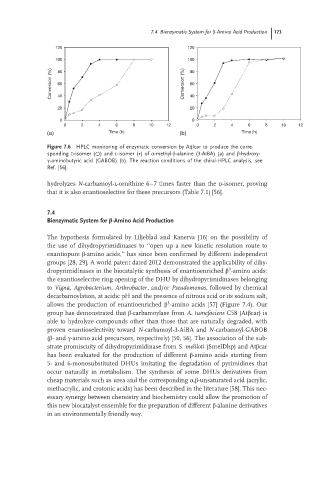
Figure 7.6 HPLC monitoring of enzymatic conversion by Atβcar to produce the corre-
sponding D-isomer (○)and L-isomer (∙)of α-methyl-β-alanine (3-AiBA) (a) and β-hydroxy-
γ-aminobutyric acid (GABOB) (b). The reaction conditions of the chiral-HPLC analysis, see
Ref. [56].
hydrolyzes N-carbamoyl-l-ornithine 6–7 times faster than the d-isomer, proving
that it is also enantioselective for these precursors (Table 7.1) [56].
7.4
Bienzymatic System for -Amino Acid Production
The hypothesis formulated by Liljeblad and Kanerva [16] on the possibility of
the use of dihydropyrimidinases to ‘‘open up a new kinetic resolution route to
enantiopure β-amino acids,’’ has since been confirmed by different independent
groups [28, 29]. A world patent dated 2012 demonstrated the applicability of dihy-
3
dropyrimidinases in the biocatalytic synthesis of enantioenriched β -amino acids:
the enantioselective ring opening of the DHU by dihydropyrimidinases belonging
to Vigna, Agrobacterium, Arthrobacter, and/or Pseudomonas, followed by chemical
decarbamoylation, at acidic pH and the presence of nitrous acid or its sodium salt,
3
allows the production of enantioenriched β -amino acids [57] (Figure 7.4). Our
group has demonstrated that β-carbamoylase from A. tumefaciens C58 (Atβcar) is
able to hydrolyze compounds other than those that are naturally degraded, with
proven enantioselectivity toward N-carbamoyl-3-AiBA and N-carbamoyl-GABOB
(β-and γ-amino acid precursors, respectively) [50, 56]. The association of the sub-
strate promiscuity of dihydropyrimidinase from S. meliloti (SmelDhp) and Atβcar
has been evaluated for the production of different β-amino acids starting from
5- and 6-monosubstituted DHUs imitating the degradation of pyrimidines that
occur naturally in metabolism. The synthesis of some DHUs derivatives from
cheap materials such as urea and the corresponding α,β-unsaturated acid (acrylic,
methacrylic, and crotonic acids) has been described in the literature [58]. This nec-
essary synergy between chemistry and biochemistry could allow the promotion of
this new biocatalyst ensemble for the preparation of different β-alanine derivatives
in an environmentally friendly way.