Page 230 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 230
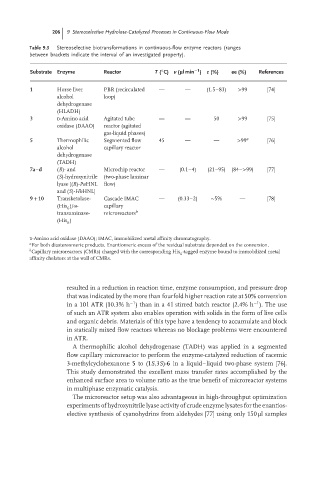
206 9 Stereoselective Hydrolase-Catalyzed Processes in Continuous-Flow Mode
Table 9.3 Stereoselective biotransformations in continuous-flow enzyme reactors (ranges
between brackets indicate the interval of an investigated property).
◦
−1
Substrate Enzyme Reactor T ( C) v ( lmin ) c (%) ee (%) References
1 Horse liver PBR (recirculated — — (1.5–83) >99 [74]
alcohol loop)
dehydrogenase
(HLADH)
3 D-Amino acid Agitated tube — — 50 >99 [75]
oxidase (DAAO) reactor (agitated
gas-liquid phases)
5 Thermophilic Segmented flow 45 — — >99 a [76]
alcohol capillary reactor
dehydrogenase
(TADH)
7a–d (R)- and Microchip reactor — (0.1–4) (21–95) (84–>99) [77]
(S)-hydroxynitrile (two-phase laminar
lyase [(R)-PaHNL flow)
and (S)-HbHNL]
9 + 10 Transketolase- Cascade IMAC — (0.33–2) ∼5% — [78]
(His )/ω- capillary
6
transaminase- microreactors b
(His )
6
D-Amino acid oxidase (DAAO); IMAC, immobilized metal affinity chromatography.
a
For both diastereomeric products. Enantiomeric excess of the residual substrate depended on the conversion.
b Capillary microreactors (CMRs) charged with the corresponding His -tagged enzyme bound to immobilized metal
6
affinity chelators at the wall of CMRs.
resulted in a reduction in reaction time, enzyme consumption, and pressure drop
that was indicated by the more than fourfold higher reaction rate at 50% conversion
−1
−1
in a 10 l ATR (10.3% h ) than in a 4 l stirred batch reactor (2.4% h ). The use
of such an ATR system also enables operation with solids in the form of live cells
and organic debris. Materials of this type have a tendency to accumulate and block
in statically mixed flow reactors whereas no blockage problems were encountered
in ATR.
A thermophilic alcohol dehydrogenase (TADH) was applied in a segmented
flow capillary microreactor to perform the enzyme-catalyzed reduction of racemic
3-methylcyclohexanone 5 to (1S,3S)-6 in a liquid–liquid two-phase system [76].
This study demonstrated the excellent mass transfer rates accomplished by the
enhanced surface area to volume ratio as the true benefit of microreactor systems
in multiphase enzymatic catalysis.
The microreactor setup was also advantageous in high-throughput optimization
experiments of hydroxynitrile lyase activity of crude enzyme lysates for the enantios-
elective synthesis of cyanohydrins from aldehydes [77] using only 150 μl samples