Page 275 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 275
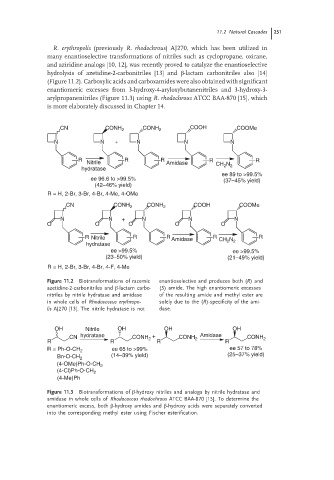
11.2 Natural Cascades 251
R. erythropolis (previously R. rhodochrous) AJ270, which has been utilized in
many enantioselective transformations of nitriles such as cyclopropane, oxirane,
and aziridine analogs [10, 12], was recently proved to catalyze the enantioselective
hydrolysis of azetidine-2-carbonitriles [13] and β-lactam carbonitriles also [14]
(Figure 11.2). Carboxylic acids and carboxamides were also obtained with significant
enantiomeric excesses from 3-hydroxy-4-aryloxybutanenitriles and 3-hydroxy-3-
arylpropanenitriles (Figure 11.3) using R. rhodochrous ATCC BAA-870 [15], which
is more elaborately discussed in Chapter 14.
CN CONH 2 CONH 2 COOH COOMe
N N + N N N
R R R R R
Nitrile Amidase CH N
hydratase 2 2
ee 89 to >99.5%
ee 96.6 to >99.5% (37–45% yield)
(42–46% yield)
R = H, 2-Br, 3-Br, 4-Br, 4-Me, 4-OMe
CN CONH 2 CONH 2 COOH COOMe
N N + N N N
O O O O O
R Nitrile R R Amidase R CH N 2 R
hydratase 2
ee >99.5% ee >99.5%
(23–50% yield) (21–49% yield)
R = H, 2-Br, 3-Br, 4-Br, 4-F, 4-Me
Figure 11.2 Biotransformations of racemic enantioselective and produces both (R)and
azetidine-2-carbonitriles and β-lactam carbo- (S) amide. The high enantiomeric excesses
nitriles by nitrile hydratase and amidase of the resulting amide and methyl ester are
in whole cells of Rhodococcus erythropo- solely due to the (R)-specificity of the ami-
lis AJ270 [13]. The nitrile hydratase is not dase.
OH Nitrile OH OH OH
CN hydratase CONH + CONH 2 Amidase CONH 2
2
R R R R
ee 65 to >99% ee 57 to 78%
R = Ph-O-CH 2
Bn-O-CH 2 (14–39% yield) (25–37% yield)
(4-OMe)Ph-O-CH 2
(4-Cl)Ph-O-CH 2
(4-Me)Ph
Figure 11.3 Biotransformations of β-hydroxy nitriles and analogs by nitrile hydratase and
amidase in whole cells of Rhodococcus rhodochrous ATCC BAA-870 [15]. To determine the
enantiomeric excess, both β-hydroxy amides and β-hydroxy acids were separately converted
into the corresponding methyl ester using Fischer esterification.