Page 280 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 280
256 11 Nitrile Converting Enzymes Involved in Natural and Synthetic Cascade Reactions
which are then subsequently converted by nitrilases or NHase/amidase systems to
the corresponding carboxylic acids (Figure 11.1).
These cascade reactions are able to convert various arylalkyl- and alkyl-aldoximes
and it was suggested that this enzymatic reaction might be useful as an environ-
mentally benign process for the synthesis of nitriles [46]. Aldoxime dehydratases
can be classified according to their substrate preferences either as aromatic or
aliphatic aldoxime dehydratases. The functional interaction of the nitrile form-
ing and converting enzymes is also reflected by the genetic organization of the
relevant genes, as the genes encoding aldoxime dehydratases and the nitrile con-
verting enzymes are often physically linked on the chromosomes of the aldoxime
degrading microorganisms. This has been demonstrated for a Bacillus strain and a
pseudomonad that harbor a combination of an aldoxime dehydratases and a nitri-
lase and also different Rhodococcus, Pseudomonas,and Acinetobacter strains, which
produce aldoxime dehydratases in combination with NHases and amidases [2, 47].
Although the triple pathway from aldoxime to acid could be attractive for chemical
synthesis, there are no examples known yet that use this cascade. This is probably
due to the fact that the aldoxime dehydratase is not very stable and the crystal
structure and mechanism of this enzyme were not elucidated until recently [46, 48].
11.2.3
Other Natural Cascades
Although most cascades described in literature are mainly academic, there are
some industrial cascades known. Lonza AG in Switzerland has been using biotrans-
formations in their organic synthesis pathways toward chiral molecules for a long
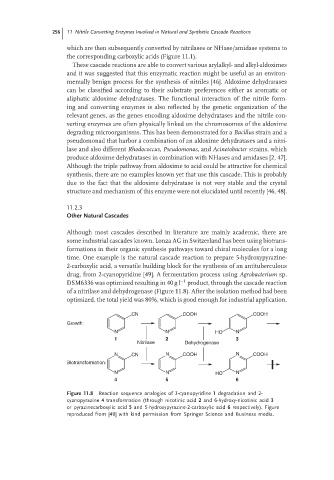
time. One example is the natural cascade reaction to prepare 5-hydroxypyrazine-
2-carboxylic acid, a versatile building block for the synthesis of an antituberculous
drug, from 2-cyanopyridine [49]. A fermentation process using Agrobacterium sp.
DSM6336 was optimized resulting in 40 g l −1 product, through the cascade reaction
of a nitrilase and dehydrogenase (Figure 11.8). After the isolation method had been
optimized, the total yield was 80%, which is good enough for industrial application.
CN COOH COOH
Growth:
N N HO N
1 2 3
Nitrilase Dehydrogenase
N CN N COOH N COOH
Biotransformation:
N N HO N
4 5 6
Figure 11.8 Reaction sequence analogies of 3-cyanopyridine 1 degradation and 2-
cyanopyrazine 4 transformation (through nicotinic acid 2 and 6-hydroxy-nicotinic acid 3
or pyrazinecarboxylic acid 5 and 5-hydroxypyrazine-2-carboxylic acid 6 respectively). Figure
reproduced from [49] with kind permission from Springer Science and Business media.