Page 284 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 284
260 11 Nitrile Converting Enzymes Involved in Natural and Synthetic Cascade Reactions
(R)- or (S)- O
Hydroxynitrile N
O
lyase HO Nitrilase HO
OH + NH 3
R 1 R 2 HCN R 1 R 2 2 H 2 O R 1 R 2
(R)- or (S)-Cyanohydrin (R)- or (S)-Carboxylic acid
H O
2
Nitrile hydratase O
H 2 Amidase
(or modified nitrilase)
O
HO
NH 2
R 1 R 2
(R)- or (S)-Carboxamide
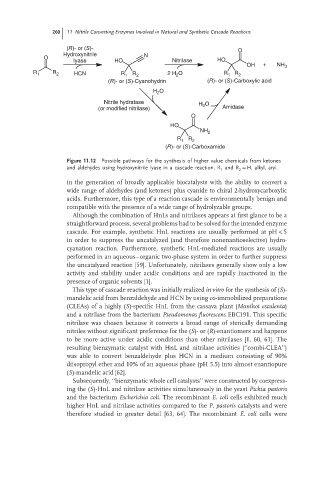
Figure 11.12 Possible pathways for the synthesis of higher value chemicals from ketones
and aldehydes using hydroxynitrile lyase in a cascade reaction. R and R = H, alkyl, aryl.
1
2
in the generation of broadly applicable biocatalysts with the ability to convert a
wide range of aldehydes (and ketones) plus cyanide to chiral 2-hydroxycarboxylic
acids. Furthermore, this type of a reaction cascade is environmentally benign and
compatible with the presence of a wide range of hydrolyzable groups.
Although the combination of HnLs and nitrilases appears at first glance to be a
straightforward process, several problems had to be solved for the intended enzyme
cascade. For example, synthetic HnL reactions are usually performed at pH < 5
in order to suppress the uncatalyzed (and therefore nonenantioselective) hydro-
cyanation reaction. Furthermore, synthetic HnL-mediated reactions are usually
performed in an aqueous–organic two-phase system in order to further suppress
the uncatalyzed reaction [59]. Unfortunately, nitrilases generally show only a low
activity and stability under acidic conditions and are rapidly inactivated in the
presence of organic solvents [1].
This type of cascade reaction was initially realized in vitro for the synthesis of (S)-
mandelic acid from benzaldehyde and HCN by using co-immobilized preparations
(CLEAs) of a highly (S)-specific HnL from the cassava plant (Manihot esculenta)
and a nitrilase from the bacterium Pseudomonas fluorescens EBC191. This specific
nitrilase was chosen because it converts a broad range of sterically demanding
nitriles without significant preference for the (S)- or (R)-enantiomers and happens
to be more active under acidic conditions than other nitrilases [1, 60, 61]. The
resulting bienzymatic catalyst with HnL and nitrilase activities (‘‘combi-CLEA’’)
was able to convert benzaldehyde plus HCN in a medium consisting of 90%
diisopropyl ether and 10% of an aqueous phase (pH 5.5) into almost enantiopure
(S)-mandelic acid [62].
Subsequently, ‘‘bienzymatic whole cell catalysts’’ were constructed by coexpress-
ing the (S)-HnL and nitrilase activities simultaneously in the yeast Pichia pastoris
and the bacterium Escherichia coli. The recombinant E. coli cells exhibited much
higher HnL and nitrilase activities compared to the P. pastoris catalysts and were
therefore studied in greater detail [63, 64]. The recombinant E. coli cells were