Page 288 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 288
264 11 Nitrile Converting Enzymes Involved in Natural and Synthetic Cascade Reactions
CN CN CN
OH OH OH
Lipase
+
OH OH OH
OH OH OAc
(±) (−) (+)
NHase
COOH COOH
COOH
CONH 2 OH OH
OH Amidase OH
O O
OH OH
OH OH OH
OH OH OH (−)
(−) (−)
(−) (−)-Cyclophellitol (−)-epi-Cyclophellitol
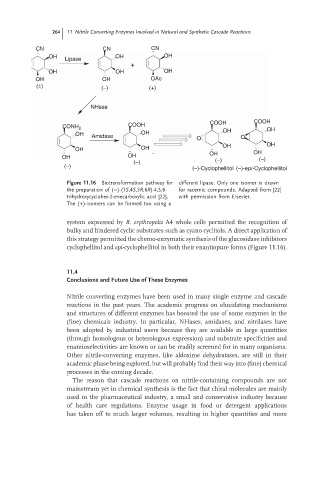
Figure 11.16 Biotransformation pathway for different lipase. Only one isomer is drawn
the preparation of (−)-(1S,4S,5R,6R)-4,5,6- for racemic compounds. Adapted from [22]
trihydroxycyclohex-2-enecarboxylic acid [22]. with permission from Elsevier.
The (+)-isomers can be formed too using a
system expressed by R. erythropolis A4 whole cells permitted the recognition of
bulky and hindered cyclic substrates such as cyano cyclitols. A direct application of
this strategy permitted the chemo-enzymatic synthesis of the glucosidase inhibitors
cyclophellitol and epi-cyclophellitol in both their enantiopure forms (Figure 11.16).
11.4
Conclusions and Future Use of These Enzymes
Nitrile converting enzymes have been used in many single enzyme and cascade
reactions in the past years. The academic progress on elucidating mechanisms
and structures of different enzymes has boosted the use of some enzymes in the
(fine) chemicals industry. In particular, NHases, amidases, and nitrilases have
been adopted by industrial users because they are available in large quantities
(through homologous or heterologous expression) and substrate specificities and
enantioselectivities are known or can be readily screened for in many organisms.
Other nitrile-converting enzymes, like aldoxime dehydratases, are still in their
academic phase being explored, but will probably find their way into (fine) chemical
processes in the coming decade.
The reason that cascade reactions on nitrile-containing compounds are not
mainstream yet in chemical synthesis is the fact that chiral molecules are mainly
used in the pharmaceutical industry, a small and conservative industry because
of health care regulations. Enzyme usage in food or detergent applications
has taken off to much larger volumes, resulting in higher quantities and more