Page 286 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 286
262 11 Nitrile Converting Enzymes Involved in Natural and Synthetic Cascade Reactions
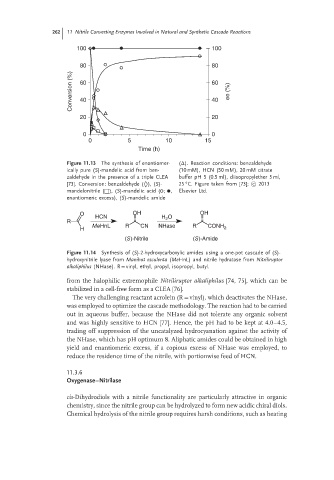
100 100
80 80
Conversion (%) 60 60 ee (%)
40
40
20 20
0 0
0 5 10 15
Time (h)
Figure 11.13 The synthesis of enantiomer- (Δ). Reaction conditions: benzaldehyde
ically pure (S)-mandelic acid from ben- (10 mM), HCN (50 mM), 20 mM citrate
zaldehyde in the presence of a triple CLEA buffer pH 5 (0.5 ml), diisopropylether 5 ml,
◦
[73]. Conversion: benzaldehyde (◊), (S)- 25 C. Figure taken from [73]; c 2013
mandelonitrile (□), (S)-mandelic acid ( ; , Elsevier Ltd.
enantiomeric excess), (S)-mandelic amide
O OH OH
HCN H O
2
R
MeHnL R CN NHase R CONH
H 2
(S)-Nitrile (S)-Amide
Figure 11.14 Synthesis of (S)-2-hydroxycarboxylic amides using a one-pot cascade of (S)-
hydroxynitrile lyase from Manihot esculenta (MeHnL) and nitrile hydratase from Nitriliruptor
alkaliphilus (NHase). R = vinyl, ethyl, propyl, isopropyl, butyl.
from the halophilic extremophile Nitriliruptor alkaliphilus [74, 75], which can be
stabilized in a cell-free form as a CLEA [76].
The very challenging reactant acrolein (R = vinyl), which deactivates the NHase,
was employed to optimize the cascade methodology. The reaction had to be carried
out in aqueous buffer, because the NHase did not tolerate any organic solvent
and was highly sensitive to HCN [77]. Hence, the pH had to be kept at 4.0–4.5,
trading off suppression of the uncatalyzed hydrocyanation against the activity of
the NHase, which has pH optimum 8. Aliphatic amides could be obtained in high
yield and enantiomeric excess, if a copious excess of NHase was employed, to
reduce the residence time of the nitrile, with portionwise feed of HCN.
11.3.6
Oxygenase–Nitrilase
cis-Dihydrodiols with a nitrile functionality are particularly attractive in organic
chemistry, since the nitrile group can be hydrolyzed to form new acidic chiral diols.
Chemical hydrolysis of the nitrile group requires harsh conditions, such as heating