Page 281 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 281
11.3 Artificial Cascades 257
Marine fungi
CN 2–8 days COOH CN
and/or
Mineral medium
124 rpm, 32 °C OH
Phenylacetic acid 2-Hydroxy-
phenylacetonitrile
100% conversion COOH
51% yield isolated
OH
2-Hydroxyphenylacetic acid
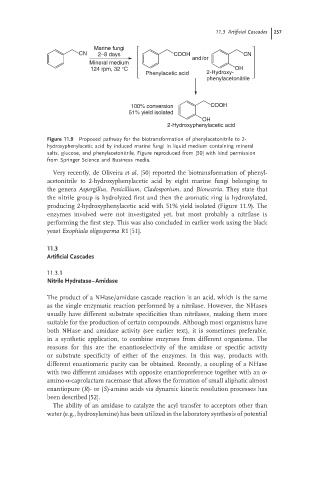
Figure 11.9 Proposed pathway for the biotransformation of phenylacetonitrile to 2-
hydroxyphenylacetic acid by induced marine fungi in liquid medium containing mineral
salts, glucose, and phenylacetonitrile. Figure reproduced from [50] with kind permission
from Springer Science and Business media.
Very recently, de Oliveira et al. [50] reported the biotransformation of phenyl-
acetonitrile to 2-hydroxyphenylacetic acid by eight marine fungi belonging to
the genera Aspergillus, Penicillium, Cladosporium,and Bionectria. They state that
the nitrile group is hydrolyzed first and then the aromatic ring is hydroxylated,
producing 2-hydroxyphenylacetic acid with 51% yield isolated (Figure 11.9). The
enzymes involved were not investigated yet, but most probably a nitrilase is
performing the first step. This was also concluded in earlier work using the black
yeast Exophiala oligosperma R1 [51].
11.3
Artificial Cascades
11.3.1
Nitrile Hydratase–Amidase
The product of a NHase/amidase cascade reaction is an acid, which is the same
as the single enzymatic reaction performed by a nitrilase. However, the NHases
usually have different substrate specificities than nitrilases, making them more
suitable for the production of certain compounds. Although most organisms have
both NHase and amidase activity (see earlier text), it is sometimes preferable,
in a synthetic application, to combine enzymes from different organisms. The
reasons for this are the enantioselectivity of the amidase or specific activity
or substrate specificity of either of the enzymes. In this way, products with
different enantiomeric purity can be obtained. Recently, a coupling of a NHase
with two different amidases with opposite enantiopreference together with an α-
amino-α-caprolactam racemase that allows the formation of small aliphatic almost
enantiopure (R)- or (S)-amino acids via dynamic kinetic resolution processes has
been described [52].
The ability of an amidase to catalyze the acyl transfer to acceptors other than
water (e.g., hydroxylamine) has been utilized in the laboratory synthesis of potential