Page 277 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 277
11.2 Natural Cascades 253
were still active. The addition of higher concentrations of ammonium sulfate
resulted in the inhibition of the amidase, making it possible to switch between the
production of benzamide or benzoic acid from benzonitrile [24].
The ability of NHases to transform a single cyano group in dinitriles provides
access to useful compounds with multiple functional groups. Dinitriles such as
malononitrile derivatives and 3-substituted glutaronitriles represent interesting
prochiral substrates in biocatalytic desymmetrization reaction because the result-
ing chiral products are often key intermediates in organic synthesis, as already
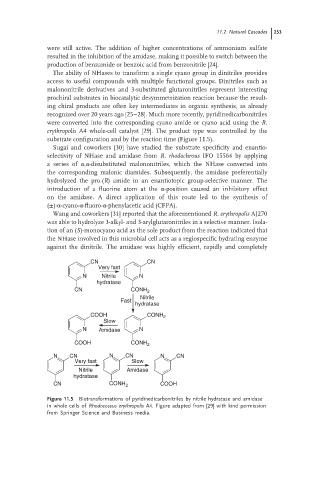
recognized over 20 years ago [25–28]. Much more recently, pyridinedicarbonitriles
were converted into the corresponding cyano amide or cyano acid using the R.
erythropolis A4 whole-cell catalyst [29]. The product type was controlled by the
substrate configuration and by the reaction time (Figure 11.5).
Sugai and coworkers [30] have studied the substrate specificity and enantio-
selectivity of NHase and amidase from R. rhodochrous IFO 15564 by applying
a series of α,α-disubstituted malononitriles, which the NHase converted into
the corresponding malonic diamides. Subsequently, the amidase preferentially
hydrolyzed the pro-(R) amide in an enantiotopic group-selective manner. The
introduction of a fluorine atom at the α-position caused an inhibitory effect
on the amidase. A direct application of this route led to the synthesis of
(±)-α-cyano-α-fluoro-α-phenylacetic acid (CFPA).
Wang and coworkers [31] reported that the aforementioned R. erythropolis AJ270
was able to hydrolyze 3-alkyl- and 3-arylglutaronitriles in a selective manner. Isola-
tion of an (S)-monocyano acid as the sole product from the reaction indicated that
the NHase involved in this microbial cell acts as a regiospecific hydrating enzyme
against the dinitrile. The amidase was highly efficient, rapidly and completely
CN CN
Very fast
N Nitrile N
hydratase
CN CONH 2
Fast Nitrile
hydratase
COOH CONH 2
Slow
N Amidase N
COOH CONH 2
N CN N CN N CN
Very fast Slow
Nitrile Amidase
hydratase
CN CONH 2 COOH
Figure 11.5 Biotransformations of pyridinedicarbonitriles by nitrile hydratase and amidase
in whole cells of Rhodococcus erythropolis A4. Figure adapted from [29] with kind permission
from Springer Science and Business media.