Page 276 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 276
252 11 Nitrile Converting Enzymes Involved in Natural and Synthetic Cascade Reactions
CN COOH
Nitrilase
R R R R
OH OH
Nitrile Amidase*
hydratase
CONH 2
R R
OH
CN CONH 2 COOH
Cl Cl Cl Cl Cl Cl
Nitrile Amidase*
hydratase
* Slow reaction
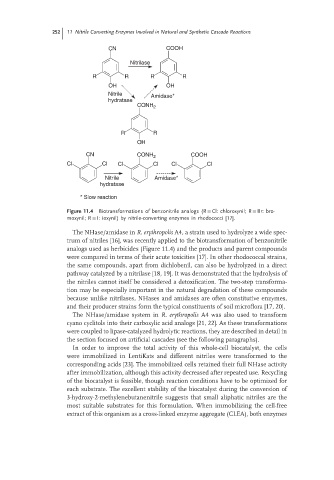
Figure 11.4 Biotransformations of benzonitrile analogs (R = Cl: chloroxynil; R = Br: bro-
moxynil; R = I: ioxynil) by nitrile-converting enzymes in rhodococci [17].
The NHase/amidase in R. erythropolis A4, a strain used to hydrolyze a wide spec-
trum of nitriles [16], was recently applied to the biotransformation of benzonitrile
analogs used as herbicides (Figure 11.4) and the products and parent compounds
were compared in terms of their acute toxicities [17]. In other rhodococcal strains,
the same compounds, apart from dichlobenil, can also be hydrolyzed in a direct
pathway catalyzed by a nitrilase [18, 19]. It was demonstrated that the hydrolysis of
the nitriles cannot itself be considered a detoxification. The two-step transforma-
tion may be especially important in the natural degradation of these compounds
because unlike nitrilases, NHases and amidases are often constitutive enzymes,
and their producer strains form the typical constituents of soil microflora [17, 20].
The NHase/amidase system in R. erythropolis A4 was also used to transform
cyano cyclitols into their carboxylic acid analogs [21, 22]. As these transformations
were coupled to lipase-catalyzed hydrolytic reactions, they are described in detail in
the section focused on artificial cascades (see the following paragraphs).
In order to improve the total activity of this whole-cell biocatalyst, the cells
were immobilized in LentiKats and different nitriles were transformed to the
corresponding acids [23]. The immobilized cells retained their full NHase activity
after immobilization, although this activity decreased after repeated use. Recycling
of the biocatalyst is feasible, though reaction conditions have to be optimized for
each substrate. The excellent stability of the biocatalyst during the conversion of
3-hydroxy-2-methylenebutanenitrile suggests that small aliphatic nitriles are the
most suitable substrates for this formulation. When immobilizing the cell-free
extract of this organism as a cross-linked enzyme aggregate (CLEA), both enzymes