Page 283 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 283
11.3 Artificial Cascades 259
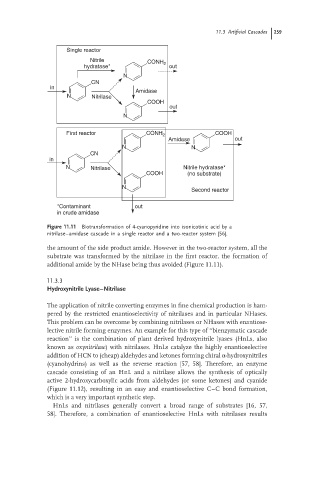
Single reactor
Nitrile
CONH 2
hydratase* out
N
CN
in
Amidase
N Nitrilase
COOH
out
N
First reactor CONH 2 COOH
Amidase out
N N
CN
in
N Nitrilase Nitrile hydratase*
COOH (no substrate)
N
Second reactor
*Contaminant out
in crude amidase
Figure 11.11 Biotransformation of 4-cyanopyridine into isonicotinic acid by a
nitrilase–amidase cascade in a single reactor and a two-reactor system [56].
the amount of the side product amide. However in the two-reactor system, all the
substrate was transformed by the nitrilase in the first reactor, the formation of
additional amide by the NHase being thus avoided (Figure 11.11).
11.3.3
Hydroxynitrile Lyase–Nitrilase
The application of nitrile converting enzymes in fine chemical production is ham-
pered by the restricted enantioselectivity of nitrilases and in particular NHases.
This problem can be overcome by combining nitrilases or NHases with enantiose-
lective nitrile forming enzymes. An example for this type of ‘‘bienzymatic cascade
reaction’’ is the combination of plant derived hydroxynitrile lyases (HnLs, also
known as oxynitrilase) with nitrilases. HnLs catalyze the highly enantioselective
addition of HCN to (cheap) aldehydes and ketones forming chiral α-hydroxynitriles
(cyanohydrins) as well as the reverse reaction [57, 58]. Therefore, an enzyme
cascade consisting of an HnL and a nitrilase allows the synthesis of optically
active 2-hydroxycarboxylic acids from aldehydes (or some ketones) and cyanide
(Figure 11.12), resulting in an easy and enantioselective C–C bond formation,
which is a very important synthetic step.
HnLs and nitrilases generally convert a broad range of substrates [16, 57,
58]. Therefore, a combination of enantioselective HnLs with nitrilases results