Page 287 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 287
11.3 Artificial Cascades 263
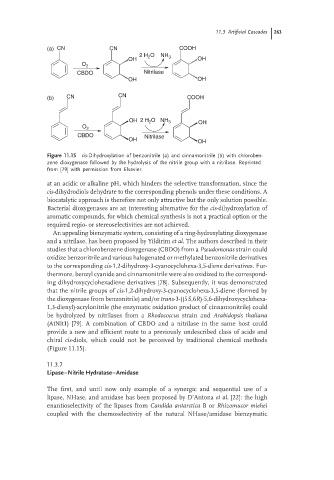
(a) CN CN COOH
2 H 2 O NH 3
OH OH
O 2
CBDO Nitrilase
OH OH
(b) CN CN COOH
OH 2 H 2 O NH 3 OH
O 2
CBDO Nitrilase
OH
OH
Figure 11.15 cis-Dihydroxylation of benzonitrile (a) and cinnamonitrile (b) with chloroben-
zene dioxygenase followed by the hydrolysis of the nitrile group with a nitrilase. Reprinted
from [79] with permission from Elsevier.
at an acidic or alkaline pH, which hinders the selective transformation, since the
cis-dihydrodiols dehydrate to the corresponding phenols under these conditions. A
biocatalytic approach is therefore not only attractive but the only solution possible.
Bacterial dioxygenases are an interesting alternative for the cis-dihydroxylation of
aromatic compounds, for which chemical synthesis is not a practical option or the
required regio- or stereoselectivities are not achieved.
An appealing bienzymatic system, consisting of a ring-hydroxylating dioxygenase
and a nitrilase, has been proposed by Yildirim et al. The authors described in their
studies that a chlorobenzene dioxygenase (CBDO) from a Pseudomonas strain could
oxidize benzonitrile and various halogenated or methylated benzonitrile derivatives
to the corresponding cis-1,2-dihydroxy-3-cyanocyclohexa-3,5-diene derivatives. Fur-
thermore, benzyl cyanide and cinnamonitrile were also oxidized to the correspond-
ing dihydroxycyclohexadiene derivatives [78]. Subsequently, it was demonstrated
that the nitrile groups of cis-1,2-dihydroxy-3-cyanocyclohexa-3,5-diene (formed by
the dioxygenase from benzonitrile) and/or trans-3-((5S,6R)-5,6-dihydroxycyclohexa-
1,3-dienyl)-acrylonitrile (the enzymatic oxidation product of cinnamonitrile) could
be hydrolyzed by nitrilases from a Rhodococcus strain and Arabidopsis thaliana
(AtNit1) [79]. A combination of CBDO and a nitrilase in the same host could
provide a new and efficient route to a previously undescribed class of acids and
chiral cis-diols, which could not be perceived by traditional chemical methods
(Figure 11.15).
11.3.7
Lipase–Nitrile Hydratase–Amidase
The first, and until now only example of a synergic and sequential use of a
lipase, NHase, and amidase has been proposed by D’Antona et al. [22]: the high
enantioselectivity of the lipases from Candida antarctica Bor Rhizomucor miehei
coupled with the chemoselectivity of the natural NHase/amidase bienzymatic