Page 342 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 342
318 15 New Applications of Transketolase: Cascade Reactions for Assay Development
O OH OH OH OH O OH OH
2− 2− TK 2− 2−
HO OPO 3 + O OPO 3 OPO 3 + HO OPO 3
ThDP, Mg 2+
OH OH O OH OH
D-X5P D-R5P D-G3P D-S7P
TPI
XK/ATP
O OH O
2− HO OPO 2−
HO OPO 3 3
OH GDH/NADH
D-Xylulose
OH
2−
HO OPO 3
L-Glycerol-3-P
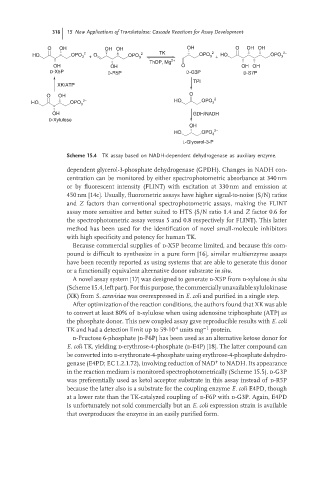
Scheme 15.4 TK assay based on NADH-dependent dehydrogenase as auxiliary enzyme.
dependent glycerol-3-phosphate dehydrogenase (GPDH). Changes in NADH con-
centration can be monitored by either spectrophotometric absorbance at 340 nm
or by fluorescent intensity (FLINT) with excitation at 330 nm and emission at
450 nm [14c]. Usually, fluorometric assays have higher signal-to-noise (S/N) ratios
and Z factors than conventional spectrophotometric assays, making the FLINT
assay more sensitive and better suited to HTS (S/N ratio 1.4 and Z factor 0.6 for
the spectrophotometric assay versus 5 and 0.8 respectively for FLINT). This latter
method has been used for the identification of novel small-molecule inhibitors
with high specificity and potency for human TK.
Because commercial supplies of d-X5P become limited, and because this com-
pound is difficult to synthesize in a pure form [16], similar multienzyme assays
have been recently reported as using systems that are able to generate this donor
or a functionally equivalent alternative donor substrate in situ.
A novel assay system [17] was designed to generate d-X5P from d-xylulose in situ
(Scheme 15.4, left part). For this purpose, the commercially unavailable xylulokinase
(XK) from S. cerevisiae was overexpressed in E. coli and purified in a single step.
After optimization of the reaction conditions, the authors found that XK was able
to convert at least 80% of d-xylulose when using adenosine triphosphate (ATP) as
the phosphate donor. This new coupled assay gave reproducible results with E. coli
-4
TK and had a detection limit up to 59⋅10 units mg −1 protein.
d-Fructose 6-phosphate (d-F6P) has been used as an alternative ketose donor for
E. coli TK, yielding d-erythrose-4-phosphate (d-E4P) [18]. The latter compound can
be converted into d-erythronate-4-phosphate using erythrose-4-phosphate dehydro-
+
genase (E4PD; EC 1.2.1.72), involving reduction of NAD to NADH. Its appearance
in the reaction medium is monitored spectrophotometrically (Scheme 15.5). d-G3P
was preferentially used as ketol acceptor substrate in this assay instead of d-R5P
because the latter also is a substrate for the coupling enzyme E. coli E4PD, though
at a lower rate than the TK-catalyzed coupling of d-F6P with d-G3P. Again, E4PD
is unfortunately not sold commercially but an E. coli expression strain is available
that overproduces the enzyme in an easily purified form.