Page 346 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 346
322 15 New Applications of Transketolase: Cascade Reactions for Assay Development
O OH OH
HO O NHAc TK O NHAc
OH O
Et CO Et
CO 2 2
D-R5P D-S7P
7 8
OH
BSA
O
HO NHAc
Et
CO 2
9
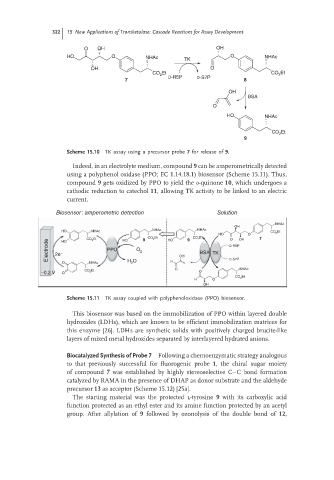
Scheme 15.10 TK assay using a precursor probe 7 for release of 9.
Indeed, in an electrolyte medium, compound 9 can be amperometrically detected
using a polyphenol oxidase (PPO; EC 1.14.18.1) biosensor (Scheme 15.11). Thus,
compound 9 gets oxidized by PPO to yield the o-quinone 10, which undergoes a
cathodic reduction to catechol 11, allowing TK activity to be linked to an electric
current.
Biosensor: amperometric detection Solution
NHAc
OH
HO NHAc NHAc NHAc CO 2 Et
HO O
CO 2 Et HO 9 CO 2 Et HO 9 CO 2 Et O OH 7
Electrode 2e − PPO O 2 OH BSA TK D-R5P
HO
O NHAc H 2 O H D-S7P
CO 2 Et O NHAc
−0.2 V O O
CO 2 Et
H O
OH
Scheme 15.11 TK assay coupled with polyphenoloxidase (PPO) biosensor.
This biosensor was based on the immobilization of PPO within layered double
hydroxides (LDHs), which are known to be efficient immobilization matrices for
this enzyme [26]. LDHs are synthetic solids with positively charged brucite-like
layers of mixed metal hydroxides separated by interlayered hydrated anions.
Biocatalyzed Synthesis of Probe 7 Following a chemoenzymatic strategy analogous
to that previously successful for fluorogenic probe 1, the chiral sugar moiety
of compound 7 was established by highly stereoselective C–C bond formation
catalyzed by RAMA in the presence of DHAP as donor substrate and the aldehyde
precursor 13 as acceptor (Scheme 15.12) [25a].
The starting material was the protected l-tyrosine 9 with its carboxylic acid
function protected as an ethyl ester and its amine function protected by an acetyl
group. After allylation of 9 followed by ozonolysis of the double bond of 12,