Page 343 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 343
15.2 Cascade Reactions for Assaying Transketolase Activity In Vitro 319
O OH OH O OH OH
2− 2−
HO OPO 3 TK HO OPO 3
2− + + 2−
OPO 3 2+ OPO 3
ThDP, Mg
OH OH O OH O OH
D-F6P D-G3P D-X5P D-E4P
E4PD/NAD +
OH
HO 2−
OPO 3
O OH
D-Erythronate-4-phosphate
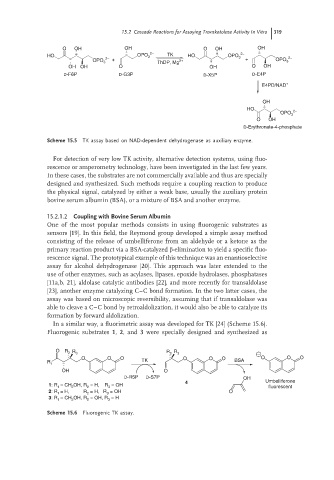
Scheme 15.5 TK assay based on NAD-dependent dehydrogenase as auxiliary enzyme.
For detection of very low TK activity, alternative detection systems, using fluo-
rescence or amperometry technology, have been investigated in the last few years.
In these cases, the substrates are not commercially available and thus are specially
designed and synthesized. Such methods require a coupling reaction to produce
the physical signal, catalyzed by either a weak base, usually the auxiliary protein
bovine serum albumin (BSA), or a mixture of BSA and another enzyme.
15.2.1.2 Coupling with Bovine Serum Albumin
One of the most popular methods consists in using fluorogenic substrates as
sensors [19]. In this field, the Reymond group developed a simple assay method
consisting of the release of umbelliferone from an aldehyde or a ketone as the
primary reaction product via a BSA-catalyzed β-elimination to yield a specific fluo-
rescence signal. The prototypical example of this technique was an enantioselective
assay for alcohol dehydrogenase [20]. This approach was later extended to the
use of other enzymes, such as acylases, lipases, epoxide hydrolases, phosphatases
[11a,b, 21], aldolase catalytic antibodies [22], and more recently for transaldolase
[23], another enzyme catalyzing C–C bond formation. In the two latter cases, the
assay was based on microscopic reversibility, assuming that if transaldolase was
able to cleave a C–C bond by retroaldolization, it would also be able to catalyze its
formation by forward aldolization.
In a similar way, a fluorimetric assay was developed for TK [24] (Scheme 15.6).
Fluorogenic substrates 1, 2,and 3 were specially designed and synthesized as
O R 2 R R
3 R 2 3
O O O O O O O O O
R TK BSA
1
OH O
D-R5P D-S7P OH
4 Umbelliferone
1: R 1 = CH 2 OH, R 2 = H, R 3 = OH
fluorescent
2: R 1 = H, R 2 = H, R 3 = OH O
3: R 1 = CH 2 OH, R 2 = OH, R 3 = H
Scheme 15.6 Fluorogenic TK assay.