Page 348 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 348
324 15 New Applications of Transketolase: Cascade Reactions for Assay Development
7000
6000
Peak area at λ = 254 nm 5000
4000
3000
2000
1000
0
0 1 2 3 4 5 6 7 8
Time (h)
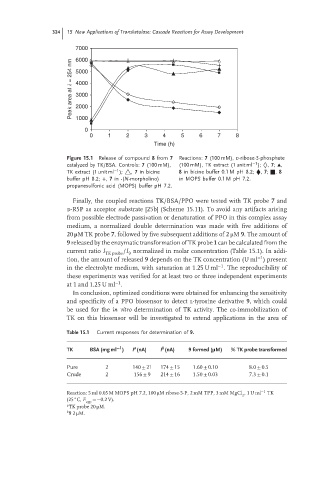
Figure 15.1 Release of compound 8 from 7 Reactions: 7 (100 mM), D-ribose-5-phosphate
−1
catalyzed by TK/BSA. Controls: 7 (100 mM), (100 mM), TK extract (1 unit ml ); ◊, 7; ▴,
−1
TK extract (1 unit ml ); △, 7 in bicine 8 in bicine buffer 0.1 M pH 8.2; ⧫, 7; ■, 8
buffer pH 8.2; +, 7 in -(N-morpholino) in MOPS buffer 0.1 M pH 7.2.
propanesulfonic acid (MOPS) buffer pH 7.2.
Finally, the coupled reactions TK/BSA/PPO were tested with TK probe 7 and
d-R5P as acceptor substrate [25b] (Scheme 15.11). To avoid any artifacts arising
from possible electrode passivation or denaturation of PPO in this complex assay
medium, a normalized double determination was made with five additions of
20 μMTKprobe 7, followed by five subsequent additions of 2 μM 9. The amount of
9 released by the enzymatic transformation of TK probe 1 can be calculated from the
current ratio I /I normalized in molar concentration (Table 15.1). In addi-
TK probe 9
−1
tion, the amount of released 9 depends on the TK concentration (U ml ) present
−1
in the electrolyte medium, with saturation at 1.25 U ml . The reproducibility of
these experiments was verified for at least two or three independent experiments
−1
at 1 and 1.25 U ml .
In conclusion, optimized conditions were obtained for enhancing the sensitivity
and specificity of a PPO biosensor to detect l-tyrosine derivative 9, which could
be used for the in vitro determination of TK activity. The co-immobilization of
TK on this biosensor will be investigated to extend applications in the area of
Table 15.1 Current responses for determination of 9.
a
b
−1
TK BSA (mg ml ) I (nA) I (nA) 9 formed ( M) % TK probe transformed
Pure 2 140 ± 21 174 ± 15 1.60 ± 0.10 8.0 ± 0.5
Crude 2 156 ± 9 214 ± 16 1.50 ± 0.03 7.3 ± 0.1
Reaction: 5 ml 0.05 M MOPS pH 7.2, 100 μM ribose-5-P, 2 mM TPP, 3 mM MgCl ,1 U ml −1 TK
2
◦
(25 C, E =−0.2 V).
app
a TK probe 20 μM.
b
9 2 μM.