Page 350 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 350
326 15 New Applications of Transketolase: Cascade Reactions for Assay Development
As a model system, experiments were carried out using (3S)-1,3-dihydroxypentan-
2-one 14, which arises from the reaction of propanal with Li-HPA in the presence
of E. coli TK. Addition of excess tetrazolium red and sodium hydroxide solution
led to a development of a red color within 2 min because of the formation of
formazane concomitantly with the diketone. The intensity displayed increased with
rising concentrations of 14. According to measurements carried out at 485 nm 14
was detectable down to a corresponding bioconversion of 2.5 mM. As an alternative
product from carboligation to benzaldehyde, l,3-dihydroxy-3-phenylpropan-2-one
15 was also tested in the assay, and comparable levels of detection were determined.
This colorimetric determination of ketose formation by a redox transformation
has several limitations. The method is restricted to nonhydroxylated aldehyde
acceptors and requires further handling steps by addition and removal of solid
reagent to eliminate residual Li-HPA. Lastly, this method, based on an endpoint
determination of the reaction product, does not allow continuous measurement of
enzyme kinetics.
15.2.2.2 Phenol Red as pH Indicator
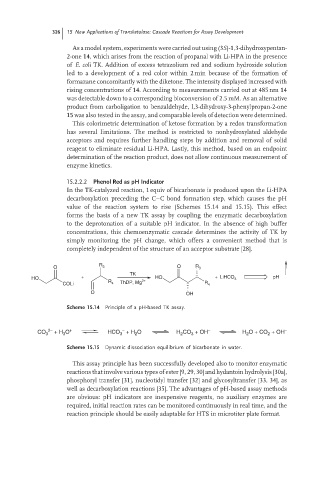
In the TK-catalyzed reaction, 1 equiv of bicarbonate is produced upon the Li-HPA
decarboxylation preceding the C–C bond formation step, which causes the pH
value of the reaction system to rise (Schemes 15.14 and 15.15). This effect
forms the basis of a new TK assay by coupling the enzymatic decarboxylation
to the deprotonation of a suitable pH indicator. In the absence of high buffer
concentrations, this chemoenzymatic cascade determines the activity of TK by
simply monitoring the pH change, which offers a convenient method that is
completely independent of the structure of an acceptor substrate [28].
R O R
O 3 3
TK
HO + 2+ HO + LiHCO 3 pH
COLi R 4 ThDP, Mg R 4
O OH
Scheme 15.14 Principle of a pH-based TK assay.
2− + − − −
CO 3 + H O HCO + H O H CO + OH H O + CO + OH
3
3
3
2
2
2
2
Scheme 15.15 Dynamic dissociation equilibrium of bicarbonate in water.
This assay principle has been successfully developed also to monitor enzymatic
reactions that involve various types of ester [9, 29, 30] and hydantoin hydrolysis [30a],
phosphoryl transfer [31], nucleotidyl transfer [32] and glycosyltransfer [33, 34], as
well as decarboxylation reactions [35]. The advantages of pH-based assay methods
are obvious: pH indicators are inexpensive reagents, no auxiliary enzymes are
required, initial reaction rates can be monitored continuously in real time, and the
reaction principle should be easily adaptable for HTS in microtiter plate format.