Page 354 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 354
330 15 New Applications of Transketolase: Cascade Reactions for Assay Development
combining a ketose moiety and the side chain of leucine or methionine, were
incubated with E. coli auxotrophs, and the growth of cells followed by their
generation time.
15.3.1
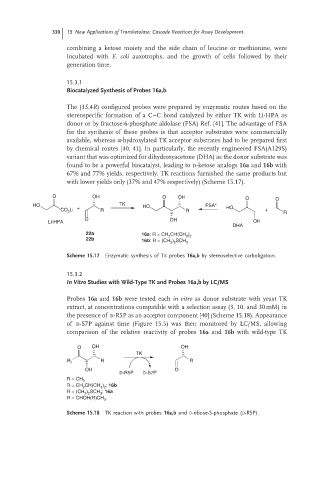
Biocatalyzed Synthesis of Probes 16a,b
The (3S,4R) configured probes were prepared by enzymatic routes based on the
stereospecific formation of a C–C bond catalyzed by either TK with Li-HPA as
donor or by fructose-6-phosphate aldolase (FSA) Ref. [41]. The advantage of FSA
for the synthesis of these probes is that acceptor substrates were commercially
available, whereas α-hydroxylated TK acceptor substrates had to be prepared first
by chemical routes [40, 41]. In particularly, the recently engineered FSA(A129S)
variant that was optimized for dihydroxyacetone (DHA) as the donor substrate was
found to be a powerful biocatalyst, leading to d-ketose analogs 16a and 16b with
67% and 77% yields, respectively. TK reactions furnished the same products but
with lower yields only (37% and 47% respectively) (Scheme 15.17).
O OH O OH O O
HO + TK HO FSA* HO
CO 2 Li R R + R
O OH
Li-HPA OH
DHA
22a 16a: R = CH CH(CH )
22b 2 3 2
16b: R = (CH 2 ) 2 SCH 3
Scheme 15.17 Enzymatic synthesis of TK probes 16a,b by stereoselective carboligation.
15.3.2
In Vitro Studies with Wild-Type TK and Probes 16a,b by LC/MS
Probes 16a and 16b were tested each in vitro as donor substrate with yeast TK
extract, at concentrations compatible with a selection assay (3, 10, and 30 mM) in
the presence of d-R5P as an acceptor component [40] (Scheme 15.18). Appearance
of d-S7P against time (Figure 15.5) was then monitored by LC/MS, allowing
comparison of the relative reactivity of probes 16a and 16b with wild-type TK
O OH OH
TK
R R R
1
OH O
D-R5P D-S7P
R = CH 3
R = CH 2 CH(CH 3 ) 2 ; 16b
R = (CH ) SCH ; 16a
2 2
3
.
R = CHOH(R)CH 3
Scheme 15.18 TK reaction with probes 16a,b and D-ribose-5-phosphate (D-R5P).