Page 358 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 358
334 15 New Applications of Transketolase: Cascade Reactions for Assay Development
leucine (Table 15.3). This could be explained by the lower efficiency for cleavage of
compound 16a by TK consistent with the results obtained in vitro, together with a
possibly inefficient transport across the bacterial cellular membrane.
Altogether, the results obtained from the present study validate the principle
of a strategy for an in vivo selection system for TK activity. In vivo,compounds
16a and 16b supplied E. coli auxotrophs with a source for the required leucine or
methionine when the bacteria expressed TK activity. However, significant growth
was recorded even in the absence of any TK gene expressed by the cells. Work
is currently in progress on the characterization of TK-independent conversion of
compound 16b, which may help to construct E. coli mutants displaying a clean or
at least strongly reduced background.
The principle of this in vivo assay could be applied to new compounds bearing
the side chain of leucine or methionine, and a sugar moiety for the selection of
TK variants with improved or modified specificities. Such compounds have already
been prepared by chemical and chemoenzymatic routes [41]. Ketoses bearing an
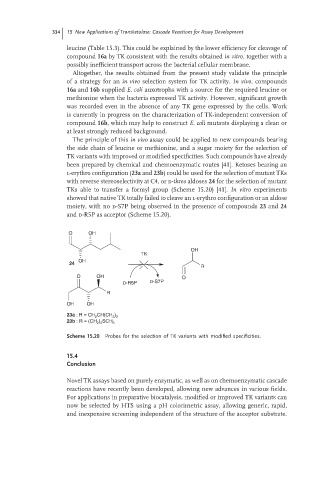
l-erythro configuration (23a and 23b) could be used for the selection of mutant TKs
with reverse stereoselectivity at C4, or d-threo aldoses 24 for the selection of mutant
TKs able to transfer a formyl group (Scheme 15.20) [41]. In vitro experiments
showed that native TK totally failed to cleave an l-erythro configuration or an aldose
moiety, with no d-S7P being observed in the presence of compounds 23 and 24
and d-R5P as acceptor (Scheme 15.20).
O OH
OH
TK
OH
24
R
O OH O
D-R5P D-S7P
R
OH OH
23a : R = CH 2 CH(CH 3 ) 2
23b : R = (CH 2 ) 2 SCH 3
Scheme 15.20 Probes for the selection of TK variants with modified specificities.
15.4
Conclusion
Novel TK assays based on purely enzymatic, as well as on chemoenzymatic cascade
reactions have recently been developed, allowing new advances in various fields.
For applications in preparative biocatalysis, modified or improved TK variants can
now be selected by HTS using a pH colorimetric assay, allowing generic, rapid,
and inexpensive screening independent of the structure of the acceptor substrate.