Page 356 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 356
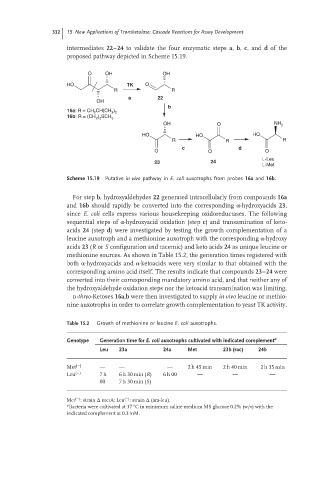
332 15 New Applications of Transketolase: Cascade Reactions for Assay Development
intermediates 22–24 to validate the four enzymatic steps a, b, c,and d of the
proposed pathway depicted in Scheme 15.19.
O OH OH
HO TK O
R R
a 22
OH
b
16a: R = CH CH(CH )
2 3 2
16b: R = (CH ) SCH 3
2 2
OH O NH 2
HO HO HO
R R R
c d
O O O
L-Leu
23 24
L-Met
Scheme 15.19 Putative in vivo pathway in E. coli auxotrophs from probes 16a and 16b.
For step b, hydroxyaldehydes 22 generated intracellularly from compounds 16a
and 16b should rapidly be converted into the corresponding α-hydroxyacids 23,
since E. coli cells express various housekeeping oxidoreductases. The following
sequential steps of α-hydroxyacid oxidation (step c) and transamination of keto-
acids 24 (step d) were investigated by testing the growth complementation of a
leucine auxotroph and a methionine auxotroph with the corresponding α-hydroxy
acids 23 (R or S configuration and racemic) and keto acids 24 as unique leucine or
methionine sources. As shown in Table 15.2, the generation times registered with
both α-hydroxyacids and α-ketoacids were very similar to that obtained with the
corresponding amino acid itself. The results indicate that compounds 23–24 were
converted into their corresponding mandatory amino acid, and that neither any of
the hydroxyaldehyde oxidation steps nor the ketoacid transamination was limiting.
d-threo-Ketoses 16a,b were then investigated to supply in vivo leucineormethio-
nine auxotrophs in order to correlate growth complementation to yeast TK activity.
Table 15.2 Growth of methionine or leucine E. coli auxotrophs.
Genotype Generation time for E. coli auxotrophs cultivated with indicated complement a
Leu 23a 24a Met 23b (rac) 24b
Met (−) — — — 2 h 45min 2h 40 min 2 h 35min
Leu (−) 7h 6h 30 min (R) 6h 00 — — —
00 7h 30 min (S)
(−)
(−)
Met :strain Δ metA; Leu :strain Δ (ara-leu).
a Bacteria were cultivated at 37 C in minimum saline medium MS glucose 0.2% (w/v) with the
◦
indicated complement at 0.3 mM.